Abstract
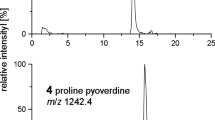
Based on a recent finding that an Azospirillum isolate ASP-1 possessing high 16S rDNA similarity to Azospirillum irakense was able to degrade desferrioxamine type siderophores (Winkelmann et al. BioMetals 9, 78-83, 1996), various members of the genus Azospirillum were analyzed for their ability to degrade desferrioxamines. While the desferrioxamine-degrading activity was absent or scarcely detectable in strains of A. lipoferum, A. brasilense, A. amazonense, degradation activity seemed to be confined to the species A. irakense (KBC-1, KA3). Also the identity of strain ASP-1 as A. irakense could be confirmed by species-specific oligonucleotide hybridization, InterLINE PCR fingerprinting and carbon source utilization pattern (BIOLOG) analysis. Products of desferrioxamine B degradation were analyzed by analytical HPLC and HPLC/electrospray mass spectrometry. Using whole cells and purified enzyme it was shown that the trihydroxamate desferrioxamine B (561 amu) is split at the N-terminal amide bond yielding a monohydroxamate (MH1, 219 amu) and a dihydroxamate (DH1, 361 amu) metabolite. A second monohydroxamate (MH2, 319 amu) resulted from DH1 after splitting the acetylhydroxamate bond. Minor amounts of a further dihydroxamate (DH2, 419 amu) originated from splitting the second amide bond in desferrioxamine B. In addition to desferrioxamine B, several other linear and cyclic desferrioxamines and derivatives were degraded, whereas desferricoprogen and desferri-ferrichrome were not degraded, indicating high substrate specificity of the desferrioxamine hydrolase in A. irakense species. A simple microtiter plate assay was developed which can be used to phenotypically discriminate and identify species of A. irakense from other Azospirillum species by their characteristic feature of desferrioxamine degradation.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990 Basic local alignment search tool. J Mol Biol 215, 403-410.
Berner I, Konetschny-Rapp S, Jung G, Winkelmann G. 1988 Characterization of ferrioxamine E as the principal siderophore of Erwinia herbicola (Enterobacter agglomerans). BioMetals 1, 51-56.
Bally R, Kabir M, Rahman M, Haurat J, Normand P 1996 Azospirillum phylogeny based on 16S rRNA sequences. In: Rahman M, ed. Biological Nitrogen Fixation Associated with Rice Production. Dordrecht: Kluwer Academic Publishers; 225-229.
Bachhawat SL, Gosh S. 1987 Iron transport in Azospirillum brasilense: Role of the siderophore spirilobactin. J Gen Microbiol 133, 1759-1765.
Ben Dekhil S, Cahill M, Stackebrandt E, Sly LI. 1997 Transfer of Conglomeromonas largomobilis subsp. largomobilis to the genus Azospirillum as Azospirillum largomobile comb. nov., and elevation of Conglomeromonas largomobilis subsp. paroonensis to the New Type Species of Conglomeromonas, Conglomeromonas parooensis sp. nov. System Appl Microbiol 20, 72-77.
Castignetti D, Siddiqui AS. 1990 The catabolism and heterotrophic nitrification of the siderophore deferrioxamine B. BioMetals 3, 197-203.
DeAngelis RM, Forsyth M, Castignetti D. 1993 The nutritional selectivity of a siderophore-catabolizing bacterium. BioMetals 6, 234-238.
Deiss K, Hantke K, Winkelmann G. 1998 Molecular recognition of siderophores: A study wioth cloned ferrioxamine receptors (FoxA) from Erwinia herbicola and Yersinia enterocolitica. BioMetals 11, 131-137.
Döbereiner J. 1992 The genera Azospirillum and Herbaspirillum. In: Ballows A, Trüper HG, Dworkin M, Harder W, Schleifer KH, eds. The Prokaryotes, 2nd edition, Vol. III, New York: Springer, 2236-2253.
Doebereiner J. 1996 Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants. In: Alef K, Nannipieri P, eds. Methods in Applied Soil Microbiology and Biochemistry London: Academic Press; 134-141.
Emery T. 1976 Fungal ornithine esterases: Relationship to iron transport. Biochemistry 15, 2723-2728.
Hartmann A. 1989 Ecophysiological aspects of growth and nitrogen fixation in Azospirillum spp. Plant Soil 110, 225-238.
Hartmann A, Gündisch C, Bode W. 1992 Azospirillum mutants improved in iron aquisition and osmotolerance as tools for the investigation of environmental fitness traits. Symbiosis 13, 271-279.
Harwani SC, Roginsky A, Vallejo Y, Castignetti D. 1997. Further characterization and proposed pathway of desferrioxamine B catabolism. BioMetals 10, 205-213.
Khammas KM, Ageron E, Grimont PAD, Kaiser P 1989 Azospirillum irakense sp. nov., a nitrogen-fixing bacterium associated with rice roots and rhizosphere soil. Res Microbiol 140, 679-693.
Kirchhof G, Hartmann A. 1992 Development of gene-probes for Azospirillum based on 23S-rRNA sequences. Symbiosis 13, 27-35.
Kirchhof G, Reis VM, Baldani JI, Eckert B, Döbereiner J, Hartmann A. 1997a Occurrence, phyiological and molecular analysis of endophytic diazotrophic bacteria in gramineous energy plants. Plant Soil 194, 45-55.
Kirchhof G, Schloter M, Aßmus B, Hartmann A. 1997b Molecular microbial ecology approaches applied to diazotrophs associated with non-legumes. Soil Biol Biochem 29, 853-862.
Leong SA, Winkelmann G 1998 Molecular biology of iron transport in fungi. In: Sigel A, Sigel H, eds, Metal Ions in Biological Systems, Vol. 35, Iron Transport and Storage in Microorganisms, Plants, and Animals. New York: Marcel Dekker, Inc.; 147-186.
Ludwig W, Dorn S, Springer N, Kirchhof G, Schleifer K-H. 1994 PCR-based preparation of 23S rRNA-targeted group-specific polynucleotide probes. Appl Environ Microbiol 60, 3236-3244.
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG 1998 Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64, 795-799.
Meyer JM, Abdallah MA. 1980 The siderochromes of non-fluorescent pseudomonads: production of nocardamine by Pseudomonas stutzeri. J Gen Microbiol 118, 125-129.
Owen RJ, Lapage SP 1976 The thermal denaturation of partly purified bacterial desoxyribonucleic acid and ist taxonomic applications. J Appl Bacteriol 41, 335-340.
Powell PE, Szaniszlo PJ, Reid CP. 1993 Confirmation of occurence of hydroxamate siderophores in soil by a novel Escherichia coli bioassay. Appl Environ Microbiol 46, 1080-1083.
Rodrigues NJ, Malavolta Va, Victot O. 1986 Meio simples para isolamento e cultivo de Xanthomonas campestris pv. citri tipo B. Suma Phytopathologica, São Paulo, Vol. 12, 1986.
Smida J, Leibhard S, Nickel AM, Eckardt-Schupp F, Hieber L. 1996. Application of repetitive sequence-based PCR (Inter LINE PCR) for the analysis of genomic rearrangements and for the genome characterization on different taxonomic levels. Gen Anal Biomol Eng 13, 95-98.
Stager CE, Davis JR 1992 Automated system for identification of microorganisms. Clin Microbiol Rev 5, 302-327.
Villavicencio M, Neilands JB. 1965 An inducible ferrichrome A-degrading peptidase from pseudomonas FC-1. Biochemistry 4, 1092-1097.
Warren RAJ, Neilands JB. 1964 Microbial degradation of the ferrichrome compounds. J Gen Microbiol 35, 459-470.
Warren RAJ, Neilands JB. 1965 Mechanism of microbial catabolism of ferrichrome A. J Biol Chem 240, 2055-2058.
Winkelmann G, Schmidtkunz K, Rainey F. 1996 Characterization of a novel Spirillum-like bacterium that degrades ferrioxamine-type siderophores. BioMetals 9, 78-83
Xia Y, Embley TM, O'Donnell AG. 1994 Phylogenetic analysis of Azospirillum by direct sequencing of PCR amplified 16SrDNA. System Appl Microbiol 17, 197-201
Zaya N, Roginsky A, Williams J, Castignetti D. 1998 Evidence that a desferrioxamine B degrading enzyme is a serine protease. Can J Microbiol 44, 1-7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winkelmann, G., Busch, B., Hartmann, A. et al. Degradation of desferrioxamines by Azospirillum irakense: Assignment of metabolites by HPLC/electrospray mass spectrometry. Biometals 12, 255–264 (1999). https://doi.org/10.1023/A:1009242307134
Issue Date:
DOI: https://doi.org/10.1023/A:1009242307134