Abstract
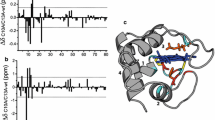
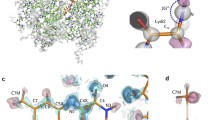
Recombinant Desulfovibrio vulgaris flavodoxin was produced inEscherichia coli. A complete backbone NMR assignment for the two-electronreduced protein revealed significant changes of chemical shift valuescompared to the oxidized protein, in particular for the flavinemononucleotide (FMN)-binding site. A comparison of homo- and heteronuclearNOESY spectra for the two redox states led to the assumption that reductionis not accompanied by significant changes of the global fold of the protein.The backbone dynamics of both the oxidized and reduced forms of D. vulgarisflavodoxin were investigated using two-dimensional15N-1H correlation NMR spectroscopy.T1, T2 and NOE data are obtained for 95%of the backbone amide groups in both redox states. These values wereanalysed in terms of the ’model-free‘ approach introduced by Lipari andSzabo [(1982) J. Am. Chem. Soc., 104, 4546-;4559, 4559-;4570]. Acomparison of the two redox states indicates that in the reduced speciessignificantly more flexibility occurs in the two loop regions enclosing FMN.Also, a higher amplitude of local motion could be found for the N(3)H groupof FMN bound to the reduced protein compared to the oxidized state.
Similar content being viewed by others
References
Abragam, A. (1961) The Principles of Nuclear Magnetism, Clarendon Press, Oxford, U.K.
Anderson, R.F. (1983) Biochim. Biophys. Acta, 722, 158-;162.
Bloom, M., Reeves, L.W. and Wells, E.J. (1965) J. Chem. Phys., 42, 1615-;1624.
Carr, H.Y. and Purcell, E.M. (1954) Phys. Rev., 94, 630-;632.
Clore, G.M., Driscoll, P.C., Wingfield, P.T. and Gronenborn, A.M. (1990a) Biochemistry, 29, 7387-;7401.
Clore, G.M., Szabo, A., Bax, A., Kay, L.E., Driscoll, P.C. and Gronenborn, A.M. (1990b) J. Am. Chem. Soc., 112, 4989-;4991.
Curley, G.P., Carr, M.C., Mayhew, S.G. and Voordouw, G. (1991) Eur. J. Biochem., 202, 1091-;1100.
Dayie, K.T. and Wagner, G. (1994) J. Magn. Reson., A111, 121-;126.
Deistung, J. and Thorneley, R.N.F. (1986) Biochem. J., 239, 69-;75.
Draper, R.D. and Ingraham, L.L. (1968) Arch. Biochem. Biophys., 125, 802-;808.
Dubourdieu, M. and Fox, J.L. (1977) J. Biol. Chem., 252, 1453-;1459.
Fushman, D., Weisemann, R., Thüring, H. and Rüterjans, H. (1994) J. Biomol. NMR, 4, 61-;78.
Grzesiek, S. and Bax, A. (1993) J. Am. Chem. Soc., 115, 12593-;12594.
Kay, L.E., Torchia, D.A. and Bax, A. (1989) Biochemistry, 28, 8972-;8979.
Kay, L.E., Nicholson, L.K., Delaglio, F., Bax, A. and Torchia, D.A. (1992) J. Magn. Reson., 97, 359-;375.
Knauf, M.A., Löhr, F., Curley, G.P., O’Farrell, P., Mayhew, S.G., Müller, F. and Rüterjans, H. (1993) Eur. J. Biochem., 213, 167-;184.
Knauf, M.A., Löhr, F., Blümel, M., Mayhew, S.G. and Rüterjans, H. (1996) Eur. J. Biochem., 238, 423-;434.
Lipari, G. and Szabo, A. (1982) J. Am. Chem. Soc., 104, 4546-;4559, 4559-;4570.
Marion, D., Driscoll, P.C., Kay, L.E., Wingfield, P.T., Bax, A., Gronenborn, A.M. and Clore, G.M. (1989) Biochemistry, 28, 6150-;6156.
Mayhew, S.G. and Ludwig, M.L. (1975) In The Enzymes, 3rd ed., Vol. 12 (Ed., Boyer, P.D.), Academic Press, New York, NY, U.S.A., pp. 57-;118.
Mayhew, S.G. and Tollin, G. (1992) In Chemistry and Biochemistry of Flavoenzymes, Vol. 3 (Ed., Müller, F.), CRC Press, Boca Raton, FL, U.S.A., pp. 389-;426.
Meiboom, S. and Gill, D. (1958) Rev. Sci. Instrum., 29, 688-;691.
Press, W.H., Flannery, B.P., Teukolsky, S.A. and Vetterling, W.T. (1988) Numerical Recipes, Cambridge University Press, Cambridge, U.K.
Simondson, R.P. and Tollin, G. (1980) Mol. Cell. Biochem., 33, 13-;24.
Vervoort, J., Müller, F., LeGall, J., Bacher, A. and Sedlmaier, H. (1985) Eur. J. Biochem., 151, 49-;57.
Vervoort, J. (1991) Curr. Opin. Struct. Biol., 1, 889-;894.
Watenpaugh, K.D., Sieker, L.C., Jensen, L.H., LeGall, J. and Dubourdieu, M. (1972) Proc. Natl. Acad. Sci. USA, 69, 3185-;3188.
Watt, W., Tulinsky, A., Swenson, R.P. and Watenpaugh, K.D. (1991) J. Mol. Biol., 218, 195-;208.
Zuiderweg, E.R.P. and Fesik, S.W. (1989) Biochemistry, 28, 2387-;2391.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hrovat, A., Blümel, M., Löhr, F. et al. Backbone dynamics of oxidized and reduced D. vulgaris flavodoxin in solution. J Biomol NMR 10, 53–62 (1997). https://doi.org/10.1023/A:1018380509735
Issue Date:
DOI: https://doi.org/10.1023/A:1018380509735