Abstract
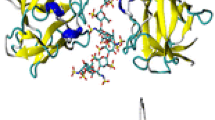
Fibroblast growth factors (FGFs) bind to extracellular matrices, especially heparin-like carbohydrates of heparansulfate proteoglycans which stabilize FGFs to protect against inactivation by heat, acid, proteolysis and oxidation. Moreover, binding of FGFs to cell surface proteoglycans promotes to form oligomers, which is essential for receptor oligomerization and activation. In the present study, we determined the solution structure of acidic FGF using a series of triple resonance multi-dimensional NMR experiments and simulated annealing calculations. Furthermore, we prepared the sample complexed with a heparin-derived hexasaccharide which is a minimum unit for aFGF binding. From the chemical shift differences between free aFGF and aFGF-heparin complex, we concluded that the major heparin binding site was located on the regions 110–131 and 17–21. The binding sites are quite similar to those observed for bFGF-heparin hexasaccharide complex, showing that both FGFs recognize heparin- oligosaccharides in a similar manner.
Similar content being viewed by others
References
Bax, A. and Pochapsky, S.S. (1992) J. Magn. Reson., 99, 638–643.
Bienkowski, M.J. and Conrad, H.E. (1985) J. Biol. Chem., 260, 356–365.
Blaber, M., DiSalvo, J. and Thomas, K.A. (1996) Biochemistry, 35, 2086–2094.
Brünger, A.T. (1993) X-PLOR Version 3.1: A System for X-Ray Crystallography and NMR, Yale University Press, New Haven, CT.
Burgess, W.H. and Maciag, T. (1989) Annu. Rev. Biochem., 58, 575–606.
Cavanagh, J., Fairbrother, W.J., Palmer III, A.G. and Skelton, N.J. (1996) Protein NMR Spectroscopy: Principles and Practice, Academic Press, San Diego, CA.
Davidson, J.M., Klagsburn, M., Hill, K.E., Buckley, A., Sullivan, R., Brewer, P.S. and Woodward, S.C. (1985) J. Cell Biol., 100, 1219–1227.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
DiGabriele, A.D., Lax, I., Chen, D.I., Svahn, C.M., Jaye, M., Schlessinger, J. and Hendrickson, W.A. (1998) Nature, 393, 812–817.
Faham, S., Hileman, R.E., Fromm, J.R., Linhardt, R.J. and Rees, D.C. (1996) Science, 271, 1116–1120.
Flaumenhaft, R., Moscatelli, D. and Rifkin, D.B. (1990) J. Cell. Biol., 111, 1651–1659.
Gospodarowicz, D. and Cheng, J. (1986) J. Cell. Physiol., 128, 475–484.
Gospodarowicz, D. (1987) Methods Enzymol., 147, 106–119.
Habuchi, H., Suzuki, S., Saito, T., Tamura, T., Harada, T., Yoshida, K. and Kimata, K. (1992) Biochem. J., 285, 805–813.
Hatanaka, H., Oka, M., Kohda, D., Tate, S., Suda, A., Tamiya, N. and Inagaki, F. (1994) J. Mol. Biol., 240, 155–166.
Iwasaki, W., Nagata, K., Hatanaka, H., Inui, T., Kimura, T., Muramatsu, T., Yoshida, K., Tasumi, M. and Inagaki, F. (1997) EMBO J., 16, 6936–6946.
Jaye, M., Burgess, W.H., Shaw, A.B. and Drohan, W.N. (1987) J. Biol. Chem., 262, 16612–16617.
Kay, L.E., Keifer, P. and Saarinen, T. (1992) J. Am. Chem. Soc., 114, 10663–10665.
Kraulis, P.J. (1991) J. Appl. Crystallogr., 24, 946–950.
Laskowski, R.A., Rullmann, J.A., MacArthur, M.W., Kaptein, R. and Thornton, J.M. (1996) J. Biomol. NMR, 8, 477–486.
Lindhal, U., Lindholt, K., Spillmann, D. and Kjellen, L. (1994) Thromb. Res., 75, 1–32.
McGee, G.S., Davidson, J.M., Buckley, A., Sommer, A., Woodward, S.C., Aquino, A.M., Barbour, R. and Demetriou, A.A. (1988) J. Surg. Res., 45, 145–153.
Moy, F.J., Seddon, A.P., Campbell, E.B., Böhlen, P. and Powers, R. (1995) J. Biomol. NMR, 6, 245–254.
Moy, F.J., Seddon, A.P., Bohlen, P. and Powers, R. (1996) Biochemistry, 35, 13552–13561.
Moy, F.J., Safran, M., Seddon, A.P., Kitchen, D., Böhlen, P., Aviezer, D., Yayon, A. and Powers, R. (1997) Biochemistry, 36, 4782–4791.
Nabel, E.G., Yang, Z-y., Plautz, G., Forough, R., Zhan, X., Haudenschild, C.C., Maciag, T. and Nabel, G.J. (1993) Nature, 362, 844–846.
Ogura, K., Terasawa, H. and Inagaki, F. (1996a) J. Biomol. NMR, 8, 492–498.
Ogura, K., Terasawa, H. and Inagaki, F. (1996b) J. Magn. Reson., B112, 63–68.
O'Keefe, E.J., Chiu, M.L. and Payner, R.E., Jr. (1988) J. Invest. Dermatol., 90, 767–769.
Pfuhl, M., Gautel, M., Politou, A.S., Joseph, C. and Pastore, A. (1995) J. Biomol. NMR, 5, 48–58.
Pineda-Lucena, A., Jiménez, M.A., Nieto, J.L., Santoro, J., Rico, M. and Giménez-Gallego, G. (1994) J. Mol. Biol., 242, 81–98.
Ruoslahti, E. and Yamaguchi, Y. (1991) Cell, 64, 867–869.
Saksela, O., Moscatelli, D., Sommer, A. and Rifkin, D.B. (1988) J. Cell. Biol., 107, 743–751.
Shaka, A.J., Lee, C.J. and Pines, A. (1988) J. Magn. Reson., 77, 274–293.
Shipley, G.D., Keeble, W.W., Hendrickson, J.E., Coffey, R.J. Jr. and Pittelkow, M.R. (1989) J. Cell. Physiol., 138, 511–518.
Sommer, A. and Rifkin, D.B. (1989) J. Cell Physiol., 138, 215–220.
Spivak-Kroizman, T., Lemmon, M.A., Dikic, I., Ladbury, J.E., Pinchasi, D., Huang, J., Jaye, M., Crumley, G., Schlessinger, J. and Lax, I. (1994) Cell, 79, 1015–1024.
Tate, S., Tate, N.U., Ravera, M.W., Jaye, M. and Inagaki, F. (1992) FEBS Lett., 297, 39–42.
Thompson, L.D., Pantoliano, M.W. and Springer, B.A. (1994) Biochemistry, 33, 3831–3840.
Thunberg, L., Backstrom, G. and Lindhal, U. (1982) Carbohydr. Res., 100, 393–410.
Vuister, G.W. and Bax, A. (1993) J. Am. Chem. Soc., 115, 7772–7777.
Wishart, D.S. and Sykes, B.D. (1994) J. Biomol. NMR, 4, 171–180.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, John Wiley, New York, NY.
Yayon, A., Klagsbrun, M., Esko, J.D., Leder, P. and Ornitz, D.M. (1991) Cell, 64, 841–848.
Zhu, X., Komiya, H., Chirino, A., Faham, S., Fox, G.M., Arakawa, T., Hsu, B.T. and Rees, D.C. (1991) Science, 251, 90–93.
Zhu, X., Hsu, B.T. and Rees, D.C. (1993) Structure, 1, 27–34.
Author information
Authors and Affiliations
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Ogura, K., Nagata, K., Hatanaka, H. et al. Solution structure of human acidic fibroblast growth factor and interaction with heparin-derived hexasaccharide. J Biomol NMR 13, 11–24 (1999). https://doi.org/10.1023/A:1008330622467
Issue Date:
DOI: https://doi.org/10.1023/A:1008330622467