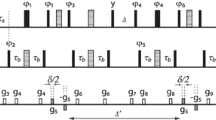
Abstract
In aqueous solution, exchanging peptide NH protons experience two environments, that of the peptide itself with a relatively slow diffusion coefficient and that of the water solvent with a faster diffusion coefficient. Although in slow exchange on the NMR chemical shift timescale, the magnetic field gradient dependence of the NH peak intensities in an experiment used to measure diffusion coefficients reflects the relative time periods spent in the two environments and this allows the determination of the relative solvent accessibility of exchangeable protons in peptides or proteins. To test this approach, the magnetic field gradient dependent intensities of the chemically shifted amide and amine NH protons of the peptide antibiotic viomycin have been measured using the high resolution longitudinal-eddy-current-delay (LED) NMR method incorporating solvent water peak elimination by non-excitation. The NH resonances of viomycin have been assigned previously and their relative exchange rates determined. Here, the gradient dependence of each NH proton intensity is reported, and these, after a bi- exponential least squares fitting, yield the fractional lifetimes of the protons spent in the peptide and water environments during the diffusion period of the experiment.
Similar content being viewed by others
References
Andrec, M. and Prestegard, J.H. (1997) J. Biomol. NMR, 9, 136–150.
Barjat, H., Morris, G.A., Smart, S., Swanson, A.G. and Williams, S.C.R. (1995) J. Magn. Reson., B108, 170–172.
Böckmann, A. and Guittet, E. (1997) FEBS Lett., 418, 127–130.
Bycroft, B.W. (1972) J. Chem. Soc. Chem. Commun., 660–661.
Chen, A., Wu, D. and Johnson, C.S. Jr. (1995) J. Am. Chem. Soc., 117, 7965–7970.
Dobson, C.M., Lian, L.-Y., Redfield, C. and Topping, K.D. (1986) J. Magn. Reson., 69, 201–209.
Gibbs, S.J. and Johnson, C.S. Jr. (1991) J. Magn. Reson., 93, 395–402.
Gozansky, E.K. and Gorenstein, D.G. (1996) J. Magn. Reson., B111, 94–96.
Grzesiek, S. and Bax, A. (1993) J. Biomol. NMR, 3, 627–638.
Hawkes, G.E., Randall, E.W., Hull, W.E., Gattegno, D. and Conti, F. (1978) Biochemistry, 17, 3986–3992.
Hinton, D.P. and Johnson, C.S. Jr. (1993) J. Phys. Chem., 97, 9064–9072.
Hrovat, M.I. and Wade, C.G. (1981) J. Magn. Reson., 44, 62–75.
Johnson, C.S. Jr. (1993) J. Magn. Reson., A102, 214–218.
Lennon, A.J., Chapman, B.E. and Kuchel, P.W. (1996) Bull. Magn. Reson., 17, 224–225.
Lennon, A.J., Scott, N.R., Chapman, B.E. and Kuchel, P.W. (1994) Biophys. J., 67, 2096–2109.
Lin, M.F. and Shapiro, M.J. (1996) J. Org. Chem., 61, 7617–7619.
Liu, M., Nicholson, J.K. and Lindon, J.C. (1996) Anal. Chem., 68, 3370–3376.
Liu, M., Parkinson, J.A., Nicholson, J.K. and Lindon, J.C. (1997) Anal. Chem., 69, 1504–1509.
Moonen, C.T.W., van Gelderen, P., Vuister, G.W. and van Zijl, P.C.M. (1992) J. Magn. Reson., 97, 419–425.
Piotto, M., Saudek, V. and Sklenar, V. (1992) J. Biomol. NMR, 2, 661–665.
Stejskal, E.O. and Tanner, J.E. (1965) J. Chem. Phys., 42, 288–292.
Stilbs, P. (1987) Prog. NMR Spectrosc., 19, 1–45.
Wu, D., Chen, A. and Johnson, C.S., Jr. (1995) J. Magn. Reson., A115, 260–264.
Wu, D., Chen, A. and Johnson, C.S., Jr. (1996a) J. Magn. Reson., A121, 88–91.
Wu, D., Chen, A. and Johnson, C.S., Jr. (1996b) J. Magn. Reson., A123, 215–218.
Wüthrich, K. (1986) NMR of Proteins and Nucleic Acids, Wiley, New York, NY.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, M., Toms, H.C., Hawkes, G.E. et al. Determination of the relative NH proton lifetimes of the peptide analogue viomycin in aqueous solution by NMR-based diffusion measurement. J Biomol NMR 13, 25–30 (1999). https://doi.org/10.1023/A:1008393202076
Issue Date:
DOI: https://doi.org/10.1023/A:1008393202076