Abstract
Emissions of nitric oxide and other odd nitrogen oxides (NO x ) from a flooded rice field were studied after urea had been broadcast into the floodwater.
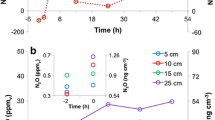
The NO x flux from the fertilized area was very low (0.2×10-9 g N m-2 s-1) for the first few days after application of urea and was high (0.95×10-9 g N m-2 s-1) in the subsequent period when significant nitrite and nitrate were present in the floodwater. At night, little if any NO x was exhaled but ambient NO2 was absorbed by the floodwater. An uptake velocity for NO2 of 3×10-4 m s-1 was measured during one night. Maximum NO x losses were observed near 1300 h when temperature and solar ultraviolet light were maximum.
While the amounts of nitrogen oxides emitted are of little agronomic importance (∼2×10-3 per cent of the fertilizer nitrogen was lost as NO x during the 10-day study period), they may well be of significance as a source for some gas reactions in the atmosphere and for the global nitrogen cycle.
Of the fertilizer nitrogen applied (as urea) approximately 30% was lost to the atmosphere by NH3 volatilization, 15% by denitrification, presumably as N2, and the remainder, less minor losses of NO and N2O, remained in the plant/soil/water system.
Similar content being viewed by others
References
Bergersen, F. J., 1980, Analysis of nitrogen by direct means, in F. J.Bergersen (ed.), Methods for Evaluating Biological Nitrogen Fixation, Wiley, Chichester, pp. 65–110.
Bolin, B. and Cook, R. B., 1983, The Major Biogeochemical Cycles and Their Interactions, Wiley, Chichester.
Bremner, J. M., 1965, Total nitrogen, in C. A.Black (ed.), Methods of Soil Analysis, Part 2, Agronomy 9, 1149–1178, Am. Soc. Agron. Madison, Wis.
Cai, G. X., Zhu, Z. L., Trevitt, A. C. F., Freney, J. R., and Simpson, J. R., 1986, Nitrogen loss from ammonium bicarbonate and urea fertilizers applied to flooded rice, Fert. Res. 10, 203–215.
Campbell, I. M., 1977, Energy and the Atmosphere, Wiley, London.
Chalk, P. M. and Smith, C. J., 1983, Chemodenitrification, in J. R.Freney and J. R.Simpson (eds.), Gaseous Loss of Nitrogen from Plant-Soil Systems, Martinus Nijhoff/Dr W. Junk Publishers, The Hague, pp. 65–89.
Crutzen, P. J., 1983, Atmospheric interactions — homogeneous gas phase reactions of C, N, and S containing compounds, in B.Bolin and R. B.Cook (eds.), The Major Biogeochemical Cycles and Their Interactions, Wiley, Chichester, pp. 67–114.
Deacon, E. L., 1977, Gas transfer to and across an air-water interface, Tellus 29, 363–374.
Delwiche, C. C., 1981, Denitrification, Nitrification and Atmospheric Nitrous Oxide, Wiley, New York.
DeMore, W. B., Margiton, J. J., Molina, M. J., Watson, R. T., Hampson, R. F., Kurylo, M. J., Howard, C. J., Ravishankara, A. R., and Golden, D. M., 1985, Chemical Kinetics and Photochemical Data for Use in Stratospheric Modelling, Evaluation No. 7, JPL Publication 85–37, NASA Jet Propulsion Laboratory, Pasadena, California.
Denmead, O. T., Simpson, J. R., and Freney, J. R., 1977, A direct field measurement of ammonia emission after injection of anhydrous ammonia, Soil Sci. Soc. Am. J., 41, 1001–1004.
Douglas, L. A. and Bremner, J. M., 1970, Extraction and colorimetric determination of urea in soils, Soil Sci. Soc. Am. Proc. 34, 859–862.
Fillery, I. R. P., Simpson, J. R., and DeDatta, S. K., 1984, Influence of field environment and fertilizer management on ammonia loss from flooded rice, Soil Sci. Soc. Am. J. 48, 914–920.
Freney, J. R., Leuning, R., Simpson, J. R., Denmead, O. T., and Muirhead, W. A., 1985, Estimating ammonia volatilization from flooded rice fields by simplified techniques, Soil Sci. Soc. Am. J. 49, 1049–1054.
Galbally, I. E., 1977, Measurement of nitrogen oxides in the background atmosphere, in Air Pollution Measurement Techniques, Special Environment Report No. 10, 179 World Meteorological Organisation, Geneva.
Galbally, I. E. and Roy, C. R., 1978, Loss of fixed nitrogen from soils by nitric oxide exhalation, Nature 275, 734–735.
Galbally, I. E. and Roy, C. R., 1980, Destruction of ozone at the earth's surface, Quart. J. Roy. Met. Soc. 106, 599–620.
Galbally, I. E., Roy, C. R., Elsworth C. M., and Rabich, H. A. H., 1985, The Measurement of Nitrogen Oxide (NO, NO 2 ) Exchange over Plant/Soil Surface, Div. Atmos. Res. Tech. Paper No. 8, CSIRO Australia.
Johansson, C. and Granat, L., 1984, Emission of nitric oxide from arable land, Tellus 36B, 25–37.
Johansson, C. and Galbally, I. E., 1984, Production of nitric oxide in loam under acrobic and anaerobic conditions, Appl. Environ. Microbiol. 47, 1284–1289.
Jorgensen, B. B., 1982, Ecology of the bacteria of the sulphur cycle with special reference to anoxicoxic interface environments, Phil. Trans. R. Soc. Lond. B 298, 543–561.
Kley, D. and McFarland, M., 1980, Chemiluminescence detector for NO and NO2, Atmospheric Technology 12, 63–69, National Centre for Atmospheric Research, Boulder, Colorado, U.S.A.
Lee, Y.-N. and Schwartz, S. E., 1981, Evaluation of the rate of uptake of nitrogen dioxide by atmospheric and surface liquid water, J. Geophys. Res. 86, 11971–11983.
Lipschultz, F., Zafiriou, O. C., Wofsy, S. C., McElroy, M. B., Valois, F. W., and Watson, S. W., 1981, Production of NO and N2O by soil nitrifying bacteria, Nature 294, 641–643.
Liss, P. S. and Slater, P. G., 1974, Flux of gases across the air-sea interface, Nature 247, 181–184.
Madronich, S., Hastie, D. R., Ridley, B. A., and Schiff, H. I., 1983, Measurement of the photodissociation coefficient of NO2 in the atmosphere: I. Method and surface measurements, J. Atmos. Chem. 1, 3–25.
Magalhães, A. M. T. and Chalk, P. M., 1986, Decomposition of methyl nitrite in solutions and soils, Soil Sci. Soc. Am. J. 50, 72–75.
McKenney, D. J., Shuttleworth, K. F., Vriesacker, J. R., and Findlay, W. I., 1982. Production and loss of nitric oxide from denitrification in anaerobic Brookston clay, Appl. Environ. Microbiol. 43, 534–541.
McKenney, D. J., Johnson, G. P., and Findlay, W. I., 1984, Effect of temperature on consecutive denitrification reactions in Brookston clay and Fox sandy loam, Appl. Environ. Microbiol. 47, 919–926.
Northcote, K. H., 1971, A Factual Key for the Recognition of Australian Soils, Rellim Tech. Publs, Glenside, S. Aust.
Orion (1983), Instruction Manual for Ammonia Electrode Model 95–12, Orion Research Inc., Cambridge, Mass.
Payne, W. J., 1981, The status of nitric oxide and nitrous oxide as intermediates in denitrification, in C. C.Delwiche (ed.), Denitrification, Nitrification and Atmospheric Nitrous Oxide, Wiley, New York, pp. 85–103.
Schwartz, S. E. and White, W. H., 1983, Kinetics of reactive dissolution of nitrogen oxides into aqueous solution, Adv. Environ. Sci. Techn. 12, 3–109.
Simpson, J. R., Freney, J. R., Wetselaar, R., Muirhead, W. A., Leuning, R., and Denmead, O. T., 1984, Transformations and losses of urea nitrogen after application to flooded rice. Aust. J. Agric. Res. 35, 189–200.
Slemr, F. and Seiler, W., 1984, Field measurements of NO and NO2 emissions from fertilized and unfertilized soils. J. Atmos. Chem. 2, 1–24.
SMSS (Soil Management Support Services), 1983, Keys to Soil Taxonomy, Technical Monograph N.6, United States Dept of Agric., Washington, D.C.
Söderlund, R. and Svensson, B. H., 1976, The global nitrogen cycle, in B. H. Svensson and R. Söderlund (eds.), Nitrogen, Phosphorus and Sulfur — Global Cycles, SCOPE Report 7, Ecol. Bull. 22, 22–73.
Taylor, W. D., Allston, T. D., Moscato, M. J., Fazekas, G. B., Kozlowski, R., and Takaes, G. A., 1980, Atmospheric photodissociation lifetimes for nitromethane, methyl nitrite and methyl nitrate, Int. J. Chem. Kinet. 12, 231–240.
White, R. J. G., 1983, CSIRO Division of Irrigation Research Ann. Report for 1981–82, CSIRO, Melbourne, Vic.
Winer, A. M., Peters, J. W., Smith, J. P., and Pitts, J. N., 1974, Response of commercial chemiluminescent NO−NO2 analyzers to other nitrogen-containing compounds, Environ. Sci. Tech. 8, 1118–1121.
Zafiriou, O. C. and McFarland, M., 1981, Nitric oxide from nitrite photolysis in the central equatorial pacific, J. Geophys. Res. 86, 3173–3182.
Author information
Authors and Affiliations
Additional information
Now at Forestry Department, Australian National University, G.P.O. Box 4, ACT 2601, Australia.
Rights and permissions
About this article
Cite this article
Galbally, I.E., Freney, J.R., Muirhead, W.A. et al. Emission of nitrogen oxides (NO x ) from a flooded soil fertilized with urea: Relation to other nitrogen loss processes. J Atmos Chem 5, 343–365 (1987). https://doi.org/10.1007/BF00114111
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00114111