Abstract
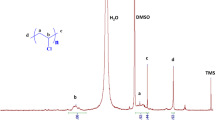
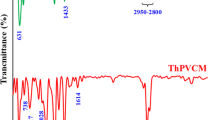
Poly(vinyl alcohol) (PVA) was reacted with strong base NaH to yield pendant oxy anions, followed with nucleophilic addition to C60. The resulted PVA(C60-Na+)n products were then converted to PVA(C60H)n by stirring with a strong acid cation exchanger of H+-form. Extraction of the C60-containing PVAs by toluene, which is a good solvent for C60, exhibits no color transfer to the toluene phase. The C60-containing PVAs were identified by the characteristic IR and UV-Vis absorptions of C60. The electrochemical behaviors in solution or in film state were investigated by cyclic voltammetric methods. The cyclic voltammogram of 4a shows a reduction peak at −2.30 V which should be due to the bonded C60 chromophores. In the film state, obtained by coating C60-containing PVA solution on graphite electrode, PVA(C60-Na+)n is much easily reduced and oxidized than PVA(C60H)n. Furthermore, the difference in this reduction and oxidation feasibility is enhanced with increasing C60 content. However, coating with PVA(C60H)n or PVA(C60-Na+)n reduces the redox ability of the graphite electrode.
Similar content being viewed by others
References
I. Amato, Science, 254, 30 (1991).
A. Hirsch, Adv. Mater., 5, 859 (1993).
J. E. Fischer, Science, 264, 1548 (1994).
R. Taylor and D. R. M. Walton, Nature, 363, 685 (1993).
F. Wudl, Acc. Chem. Res., 25, 157 (1992).
A. M. Rao, P. Zhou, K.-A. Wang, G. T. Hager, J. M. Holden, Y. Wang, W.-T. Lee, X.-X. Bi, P. C. Eklund, D. S. Cornett, M. A. Duncan and I. J. Amster, Science, 259, 955 (1993).
Y. Iwasa, T. Arima, R. M. Fleming, T. Siegrist, O. Zhou, R. C. Haddon, L. J. Rothberg, K. B. Lyons, H. L. Jr. Carter, A. F. Hebard, R. Tycko, G. Dabbagh, J. J. Krajewski, G. A. Thomas and T. Yagi, Science, 264, 1570 (1994).
Y. B. Zhao, D. M. Poirier, R. J. Pechman and J. H. Weaver, Appl. Phys. Lett., 64, 577 (1994).
H. Nagashima, A. Nakaoka, Y. Saito, M. Kato, T. Kawanishi and K. Itoh, J. Chem. Soc., Chem. Commun., 377 (1992).
P. W. Stephens, G. Bortel, G. Faigel, M. Tegze, A. Janossy, S. Pekker, G. Oszlanyi and L. Forro, Nature, 370, 636 (1994).
S. Pekker, A. Janossy, L. Mihaly, O. Chauvet, M. Carrard and L. Forro, Science, 265, 1077 (1994).
E. T. Samulski, J. M. DeSimome, M. O. Jr. Hunt, Y. Z. Menceloglu, R. C. Jarnagin, G. A. York, K. B. Labat and H. Wang, Chem. Mater., 4, 1153 (1992).
C. J. Hawker, K. L. Wooley, and J. M. J. Frechet, J. Chem. Soc., Chem. Commun., 925 (1994).
K. I. Guhr, M. D. Greaves and V. M. Rotello, J. Am. Chem. Soc., 116, 5997 (1994).
S. Shi, K. C. Khemani, Q. C. Li and F. Wudl, J. Am. Chem. Soc., 114, 10656 (1992).
K. E. Geckeler and A. Hirsch, J. Am. Chem. Soc., 115, 3850 (1993).
A. O. Patil, G. W. Schriver, B. Carstensen and R. D. Lundberg, Polym. Bull., 30, 187 (1993).
G. A. Olah, I. Bucsi, C. Lambert, R. Aniszfeld, N. J. Trivedi, D. K. Sensharma and G. K. S. Prakash, J. Am. Chem. Soc., 113, 9387 (1991).
D. A. Loy and R. A. Assink, J. Am. Chem. Soc., 114, 3977 (1992).
C. J. Hawker, Macromolecules 1994, 27, 4836.
P. J. Krusic, E. Wasserman, P. N. Keizer, J. R. Morton and K. F. Preston, Science, 254, 1183 (1991).
J. R. Morton, K. F. Preston, P. J. Krusic, S. A. Hill and E. J. Wasserman, Phys. Chem., 96, 3576 (1992).
P. J. Krusic, E. Wasserman, B. A. Parkinson, B. Malone, E. R. Holler, P. N. Keizer, J. R. Morton and K. F. Preston, J. Am. Chem. Soc., 113, 6274 (1991).
C. E. Bunker, G. E. Lawson and Y.-P. Sun, Macromolecules, 28, 3744 (1995).
E. T. Samulski, J. M. DeSimome, M. O. Jr. Hunt, Y. Z. Menceloglu, R. C. Jarnagin, G. A. York, K. B. Labat and H. Wang, Chem. Mater., 4, 1153 (1992).
R. C. Haddon, Acc. Chem. Res., 25, 127 (1992).
L. Echegoyen, Q. Xie and E. Perez-Cordero, J. Am. Chem. Soc., 114, 3978 (1995).
F. Wudl, A. Hirsch and Q. Li, Angew. Chem. Int. Ed. Engl., 30, 1309 (1991).
S. R. Wilson and Y. Wu, J. Am. Chem. Soc., 115, 10334 (1993).
K. Mikami and S. Matsumoto, Synlett., 229 (1995).
P. J. Flory, Principles of Polymer Chemistry, Chap. XIV, Cornell University Press, Ithaca, NY, 1953.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, Y., Tsai, WH. Preparation and properties of C60-containing poly(vinyl alcohol). J Polym Res 4, 17–24 (1997). https://doi.org/10.1007/s10965-006-0003-5
Issue Date:
DOI: https://doi.org/10.1007/s10965-006-0003-5