Summary
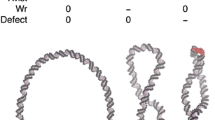
Transcriptional factors are proteins that bind to specific regulatory DNA sequences controlling the synthesis of RNA from DNA templates. Among such factors, increasing attention has been devoted to the Max gene product, the specific partner for the c-Myc oncoprotein. Productive DNA binding by Max requires dimerization, which is mediated by specific interactions of its helix-loop-helix leucine zipper binding domain. The Max-DNA complex crystallizes as a parallel, left-handed, four-helix bundle structure with the leucine zipper region forming a parallel coiled-coil. In this paper, it is shown by circular dichroism spectroscopy that the leucine zipper domain of the transcriptional factor described above (Max 75–103) is not an autonomous folding unit. The lack of dimerization function has been ascribed to the probably destabilizing effect of histidine and asparagine residues at the dimerization interface. The hydrophobic mutation of one of these amino acids (Asn92 → Val) results in the formation of a coiled-coil structure.
Similar content being viewed by others
Abbreviations
- bHLH:
-
basic helix-loop-helix
- bLZip:
-
basic leucine zipper
- CD:
-
circular dichroism
- DNA:
-
deoxyribonucleic acid
- EDT:
-
ethanedithiol
- Fmoc:
-
fluorenylmethoxycarbonyl
- GCN4:
-
general control protein 4
- HLH:
-
helix-loop-helix
- LZip:
-
leucine zipper
- HPLC:
-
high-performance liquid chromatography
- MALDI-TOF:
-
matrix-assisted laser-desorption ionization time-of-flight mass spectrometry
- MBHA:
-
4-methylbenzhydrylamine (resin)
- PAL:
-
tris(alkoxy)benzylamide linker
- PEG:
-
polyethylene glycol
- TFA:
-
trifluoroacetic acid
- TFE:
-
2,2,2,-trifluoroethanol
- Tm :
-
melting temperature at which 50% of the helicity is lost
- tR :
-
retention time
References
Murre, C., McCaw, P.S. and Baltimore, D., Cell, 56 (1989) 777.
Landschulz, W.H., Johnson, P.F. and McKnight, S.L., Science, 240 (1988) 1759.
Fisher, D.E., Parent, L.A. and Sharp, P.A., Cell, 72 (1993) 467.
Fisher, F. and Goding, C.R., EMBO J., 11 (1992) 4103.
Dushaynt, P. and Sigler, P.B., Curr. Biol., 2 (1992) 116.
Sun, X.-H. and Baltimore, D., Cell, 64 (1991) 459.
Kerpolla, T.K. and Curran, T., Curr. Biol., 1 (1991) 71.
Blackwood, E.M. and Eisenman, R.N., Science, 251 (1991) 1211.
Cole, M.D., Cell, 65 (1991) 715.
Amati, B., Brooks, M.W., Levy, N., Littlewood, T.D., Evan, G.I. and Land, H., Cell, 72 (1993) 233.
Ferré-D'Amaré, A.R., Prendergast, G.C., Ziff, E.B. and Burley, S.K., Nature, 363 (1993) 38.
Albericio, F., Kneib-Cordonier, N., Biancalana, S., Gera, L., Masada, R.I., Hudson, D. and Barany, G., J. Org. Chem., 55 (1990) 3730.
Barany, G., Albericio, F., Biancalana, S., Bontems, S., Chang, J., Eritja, R., Ferrer, M., Fields, C., Fields, G., Lyttle, M., Solé, N., Tian, Z., VanAbel, R., Wright, P., Zalipsky, S. and Hudson, D., In Smith, J.A. and Rivier, J.E. (Eds.) Peptides: Chemistry and Biology (Proceedings of the 12th American Peptide Symposium), ESCOM, Leiden, 1992, pp. 603–604.
Chen, Y.-H., Yang, J.T. and Chau, K.H., Biochemistry, 13, (1974) 3350.
Hodges, R.S., Saund, A.K., Chong, P.C.S., St.-Pierre, S.A. and Reid, R.E., J. Biol. Chem., 256 (1981) 1214.
Lau, S.Y.M., Taneja, A.K. and Hodges, R.S., J. Biol. Chem., 259 (1984) 13253.
Hodges, R.S., Semchuk, P.D., Taneja, A.K., Kay, C.M., Parker, J.M.R. and Mant, C.T., Pept. Res., 1 (1988) 19.
Hodges, R.S., Zhou, N.E., Kay, C.M. and Semchuk, P.D., Pept. Res., 3 (1990) 123.
Zhou, N.E., Kay, C.M. and Hodges, R.S., Biochemistry, 31 (1992) 5739.
Zhou, N.E., Kay, C.M. and Hodges, R.S., J. Biol. Chem., 267 (1992) 2664.
Thompson, K.S., Vinson, C.R. and Freire, E., Biochemistry, 32 (1993) 5491.
Moser, R., Protein Eng., 5 (1992) 323.
Engel, M., Williams, R.W. and Erickson, B.W., Biochemistry, 30 (1991) 3161.
Gans, P.L., Lyu, P.C., Manning, M.C., Woody, R.W. and Kallenbach, N.R., Biopolymers, 31 (1991) 1605.
Greenfield, N. and Fasman, G.D., Biochemistry, 8 (1969) 4108.
O'Shea, E.K., Klemm, J.D., Kim, P.S. and Alber, T., Science, 254 (1991) 539.
Freemont, P., Nature, 363 (1993) 20.
Su, J.Y., Hodges, R.S. and Kay, C.M., Biochemistry, 33 (1994) 15501.
Pernelle, C., Clerc, F.F., Dureuil, C., Bracco, L. and Tocque, B., Biochemistry, 32 (1993) 11682.
Sueki, M., Lee, S., Powers, S.P., Denton, J.B., Konishi, Y. and Scheraga, H.A., Macromolecules, 17 (1984) 148.
O'Neil, K.T. and DeGrado, W.F., Science, 250 (1990) 646.
Loriaux, M.M., Rehfuss, R.P., Brennan, R.G. and Goodman, R.H., Proc. Natl. Acad. Sci. USA, 90 (1993) 9046.
Harbury, P.B., Zhang, T., Kim, P.S. and Alber, T., Science, 262 (1993) 1401.
Graddis, T.J., Myszka, D.G. and Chaiken, I.M., Biochemistry, 32 (1993) 12664.
Zhu, B.Y., Zhou, N.E., Kay, C.M. and Hodges, R.S., Protein Sci., 2 (1993) 383.
O'Shea, E.K., Rutkowski, R., Stafford, W.F. and Kim, P.S., Science, 245 (1989) 646.
DeFrancesco, R., Pastore, A., Vecchio, G. and Cortese, R., Biochemistry, 30 (1991) 143.
Hurst, H.C., Protein Profile, 1 (1994) 123.
Ayer, D.E., Kretzner, L. and Eisenman, R.N., Cell, 72 (1993) 211.
Zervos, A.S., Gyuris, J. and Brent, R., Cell, 72 (1993) 223.
Author information
Authors and Affiliations
Additional information
Amino acid symbols denote the l-configuration. Abbreviations used for amino acids follow the recommendations of the IUPAC-IUB Commission of Biochemical Nomenclature [Eur. J. Biochem., 138 (1984) 9].
Rights and permissions
About this article
Cite this article
García-Echeverría, C. The leucine zipper domain of the Max gene product is not an autonomous dimerization site. Lett Pept Sci 1, 255–262 (1995). https://doi.org/10.1007/BF00127272
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00127272