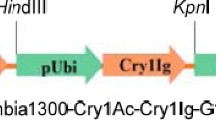
Abstract
We used particle bombardment to transform two elite Thai rice varieties, Khao Dawk Mali 105 (KDML105) and Supanburi 60 (SP60), with the snowdrop lectin gene gna (Galanthus nivalis agglutinin). This gene confers resistance to sap-sucking insects such as the brown planthopper (BPH; Nilaparvata lugens), which is one of the most damaging pests of rice. Traditionally, KDML105 and SP60 have been regarded as recalcitrant to transformation, and this is the first account of successful gene transfer to these varieties. By molecular analysis, we confirmed the recovery of over thirty gna-transgenic lines. GNA protein expression was characterised by western blot analysis, and we achieved expression levels of up to 0.25% total soluble protein. GNA-producing R1 transgenic plants were significantly more resistant to BPH than control plants (P<0.0001), with 37% and 42% reduction in nymphal survival for constitutive and phloem-specific expression, respectively. Transferring the gna gene to these superior rice varieties thus represents a major step forward for crop improvement in Thailand, and should help to reduce the damage caused by rice pests, and hence increase yields for this vital domestic and export market.
Similar content being viewed by others
References
Kisimoto R: Hopperburn injury formation of the brown plant hopper. Jpn J Plant Prot 14: 377–382 (1960).
Ou SM: Rice Diseases, 2nd ed. Commonwealth Agricultural Bureaux, Commonwealth Mycology Institute, UK (1985).
Gatehouse JA, Gatehouse AMR: Genetic engineering of plants for insect resistance. In: Reichcigl JE, Reichcigi NA (Eds.) Biological and Biotechnological Control of Insect Pests pp. 211–241. CRC Press, London (1999).
Powell KS, Gatehouse AMR, Hilder VA, Gatehouse JA: Antimetabolic effect of plant lectins and plant and fungal enzymes on the nymphal stages of two important rice pests, Nilapavata lugens and Nephotettix nigropictus. Entomol Exp Appl 66: 119–126 (1993).
Rao KV, Rathore KS, Hodges TK, Fu X, Stöger E, Sudhakar D, Williams S, Christou P, Bharathi M, Bown DP, Powell KS, Spence J, Gatehouse AMR, Gatehouse JA: Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J 15: 469–477 (1998).
Sudhakar D, Fu X, Stöger E, Williams S, Spence J, Brown DP, Bharathi M, Gatehouse JA, Christou P: Expression and immunolocalisation of the snowdrop lectin, GNA in transgenic rice plants. Transgenic Res 7: 1–8 (1998).
Christou P, Ford TL, Kofron M: Production of transgenic rice (Oryza sativa L.) plants from agronomically important indica and japonica varieties via electric discharge particle acceleration of exogenous DNA into immature zygotic embryos. Bio/technology 9: 957–962 (1991).
Hiei Y, Ohta S, Komari T, Kumashiro T: Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 (1994).
Fu X, Li D, Christou P: Vectors construction of snowdrop lectin (GNA) gene and expression in transgenic rice. J Zhejiang Agric Univ 23: 730–731 (1997).
Potrykus I, Harms CT, Lörz H: Callus formation from cell culture protoplasts of cron (Zea mays L.) Theor Appl Genet 54: 209–214 (1979).
Hartke S, Lörz H: Somatic embryogenesis and plant regeneration from various indica rice genotypes (Oryza sativa L.) J Genet Breed 43: 205–214 (1989).
Sudhakar D, Duc LT, Bong BB, Tinjuangjun P, Maqbool SB, Valdez M, Jefferson AR, Christou P: An efficient rice transformation system utilising mature seed-derived explants and a portable, inexpensive particle bombardment device. Transgenic Res 7: 289–294 (1998).
Edwards K, Johnston C, Thompson C: A simple and rapid method for the preparation of plant genomic DNA for PCR analyses. Nucl Acids Res 98: 1349 (1991).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: ?-glucuronidase as a sensitive and versatile gene fusion marker in higher plants EMBO J 6: 3901–3907 (1987)
Laemmli EK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Stöger E, Williams S, Christou P, Down RE, Gatehouse JA: Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin; GNA) in transgenic wheat plants: effect on predation by the grain aphid Sitobion avenae. Mol Breed 5: 65–73 (1999).
Khush GS: Origin, dispersal, cultivation and variation of rice. In: Sasaki T, Moore G (Eds.) Oryza: From Molecule to Plant, pp. 25–34. Kluwer Academic Publishers, Dordrecht, The Netherlands (1997).
Setboonsarng S: Rice research priorities in Thailand. In: Evenson RE, Herdt RW, Hossain M (Eds.) Rice Research in Asia: Progress and Priorities, pp. 217–238. CAB International, Wallingford, UK (1996).
Gatehouse AMR: Biotechnological applications of plant genes in the production of insect-resistant crops. In: Clement SL, Quisenberry SS (Eds.) Global Plant Genetic Resources for Insect-Resistant Crops, pp. 263–280. CRC Press, London (1999).
Vajrabhaya M, Tunvachkul O, Vajrabhaya T: Effect of auxin and cytokinin on plant regeneration from rice callus. J Sci Res Chula Univ 11: 113–115 (1986).
Vajrabhaya M, Thanapaisal T, Vajrabhaya T: Development of salt tolerant lines of KDML and LPT rice cultivars through tissue culture. Plant Cell Rep 8: 411–414 (1989).
Poonsapaya P, Nabors MW, Wright K, Vajrabhaya M: A comparison of methods for callus culture and plant regeneration of RD25 rice (Oryza sativa L.) in two laboratories. Plant Cell Tissue Organ Cult 16: 175–186 (1989).
Kunanuvatchaidach R, Godwin ID, Adkins SW: High efficiency plant regeneration from callus induced on mature indica rice caryopses. Aust J Bot 43: 337–348 (1995).
Vain P, Worland B, Clarke MC, Richard g, Beavis M, Liu H, Kohli A, Leech M, Snape J, Christou P, Atkinson H: Expression of an engineered cysteine proteinase inhibitor (Oryzacystatin-I1D86) for nematode resistance in transgenic rice plants. Theor Appl Genet 96: 266–271 (1998).
Valdez M, Cabrera Ponce JL, Sudhakar D, Herrera Estrella I, Christou P: Transgenic central American, West African and Asian elite rice varieties resulting from particle bombardment of foreign DNA into mature seed-derived explants utilizing three different bombardment devices. Ann Bot 82: 795–801 (1989).
Kohli A, Gahakwa D, Vain P, Laurie DA, Christou P: Transgene expression in rice engineered through particle bombardment: molecular factors controlling stable expression and transgene silencing. Planta 208: 88–97 (1999).
Stöger E, Williams S, Keen D, Christou P: Molecular characteristics of transgenic wheat and the effect on transgene expression. Transgenic Res 7: 463–471 (1998).
Tang K, Tinjuangjun P, Xu Y, Sun X, Gatehouse JA, Ronald PC, Qi H, Lu X, Christou P, Kohli A: Particle bombardment-mediated co-transformation of elite Chinese rice cultivars with genes conferring resistance to bacterial blight and sap sucking insect pests. Planta 208: 552–563 (1999).
Goto F, Toki S, Uchimiya H: Inheritance of a co-transferred foreign gene in the progenies of transgenic rice plants. Transgenic Res 2: 302–305 (1993).
Gatehouse AMR, Down RE, Powell KS, Sauvion N, Rahbe Y, Newell CA, Merryweather A, Gatehouse JA: Effects of GNA-expressing transgenic potato plants on peach-potato aphid, Myzus persicae. Entomol Exp Appl 79: 295–307 (1996).
Down RE, Gatehouse AMR, Hamilton WDO, Gatehouse JA: Snowdrop lectin inhibits development and fecundity of the glasshouse potato aphid (Aulacorthum solani) when administered in vitro and via transgenic plants both in laboratory and glasshouse trials. J Insect Physiol 42: 1035–1045 (1996).
Foissac X, Loc NT, Christou P, Gatehouse AMR, Gatehouse JA: Resistance to green leafhopper (Nephotetix virescens) and brown planthopper (Nilaparvata lugens) in transgenic rice expressing snowdrop lectin (Galanthus nivalis agglutinin; GNA), J Insect Physiol 46: 573–583 (2000).
Harper SM, Crenshaw RW, Mullins MA, Privalle LS: Lectin binding to insect brush boarder membranes. J Econ Entomol 88: 1197–1202 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tinjuangjun, P., Loc, N., Gatehouse, A. et al. Enhanced insect resistance in Thai rice varieties generated by particle bombardment. Molecular Breeding 6, 391–399 (2000). https://doi.org/10.1023/A:1009633703157
Issue Date:
DOI: https://doi.org/10.1023/A:1009633703157