Summary
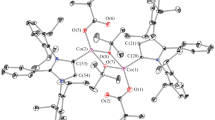
Reaction of CoII with N(CN) sup−inf2 or C(CN) sup−inf3 in the presence of imidazole (iz) or its methyl derivatives (2-meiz and 4-meiz) gave eight compounds of CoII: ligand stoichiometry 1∶2, including two isomeric pairs (α and β) for the complexes [Co{C(CN)3}2(2-meiz)2] and [Co{C(CN)3}2-(4-meiz)2]. The complexes were studied by electronic and i.r. spectroscopies. For α-[Co{C(CN)3}2(2-meiz)2] singlecrystal X-ray analysis was performed; its crystal structure consists of one-dimensional chains, formed by C(CN) sup−inf3 anions bridging between the CoII atoms. The CoII atom is nearly octahedrally coordinated by two tertiary nitrogens of 2-meiz and four nitrogens of C(CN) sup−inf3 . The spectra of these compounds and of the complexes with iz, as well as that of α-[Co{C(CN)3}2(4-meiz)2], indicate all these compounds to have basically the same bridging polymeric octahedral structure. However, the spectra indicate distorted tetrahedral structures for the remaining compounds.
Similar content being viewed by others
References
M. Hvastijová, J. Kohout, M. Okruhlica, J. Mroziński and L. Jäger,Transition Met. Chem.,18, 579 (1993).
M. Hvastijová, J. Kohout, J. Mroziński and L. Jäger,Polish J. Chem., accepted.
J. Mroziński, M. Hvastijová and J. Kohout,Polyhedron,11, 2867 (1992).
G. M. Sheldrick,Acta Crystallogr.,A46, 467 (1990).
G. M. Sheldrick,SHELXL93. Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1993.
M. Hvastijová, J. Kohout, H. Wusterhausen and H. Köhler,Z. Anorg. Allg. Chem.,434, 29 (1977).
C. K. Johnson,ORTEPII. Report ORNL-3794, revised. Oak Ridge National Laboratory, TN, USA, 1971.
M. Nardelli,Comput. Chem.,7, 95 (1983).
A. B. P. Lever,Inorganic Electronic Spectroscopy, 2nd Edit., Elsevier, Amsterdam, 1984, p. 480.
H. Köhler in A. M. Golub, H. Köhler and V. V. Skopenko (Eds.),Chemistry of Pseudohalides, Elsevier, Amsterdam, 1986, p. 413.
M. R. Grimmet in K. T. Potts (Ed.),Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxford, 1984, Vol. 5, p. 383.
A. A. G. Tomlinson, C. Bellitto, O. Piovesana and C. Furlani,J. Chem. Soc., Dalton Trans., 350 (1972)
J. M. Land, J. A. Stubbs and J. T. Wrobleski,Inorg. Chem.,16, 1955 (1977).
H. Köhler and B. Seifert,Z. Anorg. Allg. Chem.,352, 265 (1967).
H. Köhler,Z. Chem.,13, 401 (1973)
H. Köhler,Koord. Khim.,3, 139 (1977).
M. de N. D. Cordes and J. L. Walter,Spectrochim. Acta,24A, 237 (1968)
C. Perchard and A. Novak,Spectrochim. Acta,23A, 1953 (1967)
M. Hvastijová and J. Kohout, Unpublished work.
R. J. H. Clark and C. S. Williams,Inorg. Chem.,4, 350 (1965).
R. J. H. Clark and C. S. Williams,Spectrochim. Acta,22, 1081 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hvastijová, M., Kožísek, J., Kohout, J. et al. X-ray crystallographic and physical investigation of tricyanomethanide and dicyanamide complexes of cobalt(II) with imidazole and its methyl derivatives. Transition Met Chem 20, 276–279 (1995). https://doi.org/10.1007/BF00143492
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00143492