Abstract
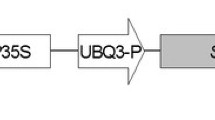
With the aim of increasing the rumen-protected level of the sulphur amino acids cysteine and methionine in Trifolium repens, we introduced the coding sequence of the sunflower seed albumin (SSA) into T. repens by Agrobacterium tumefaciens-mediated transformation. The SSA gene was modified such that the protein would be localised to the endoplasmic reticulum (ER). Four different T-DNA constructions all containing the SSA gene driven by either the promoter of a gene encoding the small subunit of ribulose bisphosphate carboxylase (Rubisco) from Arabidopsis thaliana (A ssu), the promoter of the gene encoding the small subunit of Rubisco of Medicago sativa (L ssu), or the Cauliflower Mosaic Virus 35S promoter (CaMV35S), were transferred to T. repens cv. Haifa. Transgenic T 0-plants and inter-transgenic hybrids were analysed for the level of SSA accumulation in the leaves by western blotting. The highest observed level of SSA accumulation was 0.1% of total extractable leaf protein. We observed that the promoter had a substantive effect on the level of SSA accumulation with A ssu>CaMV35S>L ssu. Results from the inter-transgenic hybrids showed that the capacity to synthesise SSA was inherited. However the level of SSA accumulation in the leaves generally appears not to be additive with extra transgenic loci. During this work, we attempted to improve the efficiency of A. tumefaciens-mediated transformation of T. repens using the SAAT-method (Sonication Assisted Agrobacterium-mediated Transformation) on cotyledons of T. repens. T-DNA transfer was in general not enhanced by sonication compared to traditional A. tumefaciens-mediated transformation. Furthermore, Southern blot analyses of plants regenerated from the same cotyledon after A. tumefaciens treatment and under selection, indicated that multiple shoots were usually derived from the same transformation event. We concluded from these results that only one plant from each A. tumefaciens-treated cotyledon should be taken to avoid transgenic clones.
Similar content being viewed by others
References
An G, Ebert PR, Mitra A and Ha SB (1988) Binary vectors. In: Gelvin SB, Schilperoort RA and Verma DPS (eds) Plant Mol Biol Manual, suppl. 6, Kluwer, Dordrecht, A3/1–A3/19.
An G, Watson BD, Stachel S, Gordon MP and Nester EW (1985) New cloning vehicles for transformation of higher plants. EMBO J 4: 277–284.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
Cashmore AR (1983) Nuclear genes encoding the small subunit of ribulose bisphosphate carboxylase. In: Kosuge T, Meredith CP and Hollaender A (eds.) Genetic Engineering of Plants, (pp. 29–38), Plenum Press, New York.
De Block M, Botterman J, Vandewiele M, Dockx J, Thoen C, Gosselé V, Rao Movva N, Thompson C, Van Montagu M and Leemans J (1987) Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J 6: 2513–2518.
Ealing PM, Hancock KR and White DWR (1994) Expression of the pea albumin 1 gene in transgenic white clover and tobacco. Transgenic Res 3: 344–354.
Gamborg OL and Eveleigh DE (1968) Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can J Biochem 46: 414–421.
Garfinkel DJ and Nester EW (1980) Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol 144: 732–743.
Gheysen G, Villarroel R and Van Montagu M (1991) Illegitimate recombination in plants: a model for T-DNA integration. Genes Dev 5: 287–297.
Hondred D, Walker JM, Mathews DE and Vierstra RD (1999) Use of ubiquitin fusions to augment protein expression in transgenic plants. Plant Physiol 119: 713–23.
Janssen BJ and Gardner RC (1989) Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol 14: 61–72.
Jefferson RA, Kavanagh TA and Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907.
Jones JDG, Shlumukov L, Carland F, English J, Scofield SR, Bishop GJ and Harrison K (1992) Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res 1: 285–297.
Jorgensen R, Snyder C and Jones JDG (1987) T-DNA is organized predominantly in inverted repeat structures in plants transformed with Agrobacterium tumefaciens C58 derivatives. Mol Gen Genet 207: 471–477.
Khan MRI, Ceriotti A, Tabe L, Aryan A, McNabb W, Moore A, Craig S, Spencer D and Higgins TJV (1996) Accumulation of a sulphur-rich seed albumin from sunflower in the leaves of transgenic subterranean clover (Trifolium subterraneum L.). Transgenic Res 5: 179–185.
Kortt AA, Caldwell JB, Lilley GG and Higgins TJV (1991) Amino acid and cDNA sequences of a methionine-rich 2S protein from sunflower seed (Helianthus annuus L.). Eur J Biochem 195: 329–334.
Langlands JP (1970) Efficiency of wool production of grazing sheep 3. The use of sulphur-containing amino acids to stimulate wool growth. Aust J Exp Anim Husb 10: 665–671.
Larkin PJ, Gibson JM, Mathesius U, Weinman JJ, Gartner E, Hall E, Tanner GJ, Rolfe BG and Djordjevic MA (1996) Transgenic white clover. Studies with the auxin responsive promoter, GH3, in root gravitropism and lateral root development. Transgenic Res 5: 325–335.
Lazo GR, Stein PA and Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Bio/Tech 9: 963–967.
Liebholz J (1984) A note on methionine supplementation of pig grower diets containing lupin-seed meal. Anim Prod 38: 515–517.
Mayerhofer R, Koncz-Kalman Z, Nawrath C, Bakkeren G, Crameri A, Angelis K, Redei GP, Schell J, Hohn B and Koncz C (1991) TDNA integration: a mode of illegitimate recombination in plants. EMBO J 10: 697–704.
McNabb WC, Spencer D, Higgins TJ and Barry T (1994) In vitro rates of rumen proteolysis of ribulose-1,5-bisphosphate carboxylase (rubisco) from lucerne leaves, and of ovalbumin, vicilin and sunflower albumin 8 storage proteins. J Sci Food Agric 64: 53–61.
Meurer CA, Dinkins RD and Collins GB (1998) Factors affecting soybean cotyledonary node transformation. Plant Cell Rep 18: 180–186.
Molvig L, Tabe LM, Eggum BO, Moore AE, Craig S, Spencer D and Higgins TJV (1997) Enhanced methionine levels and increased nutritive value of seeds of transgenic lupins (Lupinus angustifolius) expressing a sunflower seed albumin gene. Proc Natl Acad Sci USA 94: 8393–8398.
Munro S and Pelham HRB (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48: 899–907.
Odell JT, Nagy F and Chua NH (1984) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812.
Phillips GC and Collins GB (1984) Red clover and other forage legumes. In: Sharp WR, Evans DA, Amanatol PV and Yamada Y (eds.) Handbook of Plant Cell Culture 2, Crop Species, (pp. 169–210) Macmillan, New York.
Pickering FS and Reis PJ (1993) Effects of abomasal supplements of methionine on wool growth of grazing sheep. Aust J Exp Agri 33: 7–12.
Pietrzak M, Shillito RD, Hohn T and Potrykus I (1986) Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucl Acids Res 14: 5857–5868.
Potrykus I (1990) Gene transfer to cereals: An assessment. Bio/Tech 8: 535–542.
Sambrook J, Fritsch EF and Maniatis T (1989) Molecular Cloning: A Laboratory Manual. 2nd edition, (pp. 9.31–9.62) Cold Spring Harbor Laboratory, USA.
Santarém ER, Trick HN, Essig JS and Finer JJ (1998) Sonication-assisted Agrobacterium-mediated transformation of soybean immature cotyledons: optimization of transient expression. Plant Cell Reports 17: 752–759.
Scott A, Woodfield D and White DWR (1998) Allelic composition and genetic background effects on transgene expression and inheritance in white clover. Mol Breed 4: 479–490.
Sharma SB, Hancock KR, Ealing PM and White DWR (1998) Expression of a sulfur-rich maize seed storage protein, δ-zein, in white clover (Trifolium repens) to improve forage quality. Mol Breed 4: 435–448.
Spencer TM, Gordon-Kamm WJ, Daines RJ, Start WG and Lemaux PG (1990) Bialophos selection of stable transformants from maize cell culture. Theor Appl Genet 79: 625–631.
Svab Z and Maliga P (1993) High frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90: 913–917.
Tabe LM, Higgins CM, McNabb WC and Higgins TJV (1993) Genetic engineering of grain and pasture legumes for improved nutritive value. Genetica 90: 181–200.
Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A and Higgins TJV (1995) A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci 73: 2752–2759.
Taylor NL (1985) Clover Science and Technology, (pp. 1–5) American Society of Agronomy, Crop Science Society of America, Soil science Society of America, Publishers, Madison, WI, USA.
Thompson CJ, Rao Movva N, Tizard R, Crameri R, Davies JE, Lauwereys M and Botterman J (1987) Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J 6: 2519–2523.
Trick HN and Finer JJ (1997) SAAT: Sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6: 329–336.
Trick HN and Finer JJ (1998) Sonication assisted Agrobacterium mediated transformation of soybean (Glycine max (L.) Merrill) embryogenic suspension culture tissue. Plant Cell Reports 17: 482–488.
Trick HN and Finer JJ (1999) Induction of somatic embryogenesis and genetic transformation of Ohio buckeye (Aesculus glabra Willd.). In Vitro Cell Devel Biol Plant 35: 57–60.
Villemont E, Dubois F, Sangwan RS, Vasseur G, Bourgeois Y and Sangwan-Norreel BS (1997) Role of the host cell cycle in the Agrobacterium-mediated genetic transformation of Petunia: evidence of an S-phase control mechanism for T-DNA transfer. Planta 201: 160–172.
Voisey CR, White DWR, Dudas B, Appleby RD, Ealing PM and Scott AG (1994) Agrobacterium-mediated transformation of white clover using direct shoot organogenesis. Plant Cell Rep 13: 309–314.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christiansen, P., Gibson, J., Moore, A. et al. Transgenic Trifolium repens with foliage accumulating the high sulphur protein, sunflower seed albumin. Transgenic Res 9, 103–113 (2000). https://doi.org/10.1023/A:1008967409302
Issue Date:
DOI: https://doi.org/10.1023/A:1008967409302