Abstract
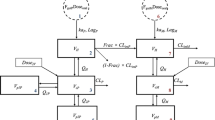
The pharmacokinetics of a slow-release theophylline formulation was investigated following intravenous and oral administration at 10 mg/kg in horses. A tricompartmental model was selected to describe the intravenous plasma profile. The elimination half-life (t1/2β) was 16.91 ± 0.93 h, the apparent volume of distribution (V d) was 1.35 ± 0.18 L/kg and the body clearance (ClB) was 0.061 ± 0.009 L kg−1 h. After oral administration the half-life of absorption was 1.24 ± 0.30 h, and the calculated bioavailability was above 100%. Thet1/2β after oral administration was 18.51 ± 1.75 h, only a little longer than that after intravenous administration. The slow release formulation did not exhibit any advantage in prolonging thet1/2β of theophylline in the horse.
Similar content being viewed by others
References
Akaike, H., 1976. An information criterion (AIC).Mathematical Science,14, 5–9
Ayres, J., Pearson, E., Riebold, T. and Chang, S., 1985. Theophylline and diphylline pharmacokinetics in the horse.American Journal of Veterinary Research,46, 2500–2506
Beech, H., 1979. Principles of therapy.Veterinary Clinics of North America. Large Animals Practice,1, 73–88
D'Argenio, D. and Schumitzky, A., 1979. A program package for simulation and parameter estimation in pharmacokinetic systems.Computer Programs in Biomedicine,9, 115–134
Davis, L.E., 1980. Applied pharmacodynamics of digitalis, theophylline, diuretics and selected antiarrythmics drugs. In: R.W. Kirk (ed),Current Veterinary Therapy VII. Small Animal Practice, (W.B. Saunders, Philadelphia), 352–359
Davis, L.E., Neff Davis, C., Koritz, G.D., Bevill, R.F., Sharma, G.C., Langston, V.C. and Munsiff, I.J., 1987. Effect of organic vehicles in pharmacokinetics of aminophylline administered intravenously to goats.Journal of Veterinary Pharmacology and Therapeutics,10, 144–149
Derksen, F.J., 1983. Chronic airway disease. In: E. Robinson (ed),Current Therapy in Equine Medicine, (W.B. Saunders, Philadelphia), 509
Errecalde, J.O., Button, C., Baggot, J.D. and Mulders, M.S.G., 1984. Pharmacokinetics and bioavailability of theophylline in horses.Journal of Veterinary Pharmacology and Therapeutics,7, 255–264
Hendeles, L. and Weinberger, M., 1982. Improved efficacy and safety of theophylline in the control of airways hyperactivity.Annals of Allergy,49, 247–256
Ingvast-Larson, C., Paalzow, L., Ottosson, T. and Appelgreen, L.E., 1985. Pharmacokinetic studies of theophylline in horses.Journal of Veterinary Pharmacology and Therapeutics,8, 76–81
Jacobs, M.H., Senior, R.M. and Kessler, G., 1976. Clinical experience with theophylline: relationship between dosage, serum concentration and toxicity.Journal of the American Medical Association,235, 1983–1986
Jenne, J.W., Wyze, E., Rood, F.S. and McDonald, F.M., 1972. Pharmacokinetics of theophylline: application to adjustment of the clinical dose of theophylline.Clinical Pharmacology and Therapeutics,13, 349–360
Koritz, G., Borne, D., Hunt, J. and Prasad, V., 1981. Pharmacokinetics of theophylline in swine: a potential model for human drug bioavailability studies.Journal of Veterinary Pharmacology and Therapeutics,4, 233–239
Koritz, G., McKiernan, B. and Neff Davies, C., 1986. Bioavailability of four slow release formulations in the Beagle dog.Journal of Veterinary Pharmacology and Therapeutics,9, 293–302
Kowalczyk, D.F., Beech, J. and Littlejohn, D., 1984. Pharmacokinetic disposition of theophylline in horses after intravenous administration.American Journal of Veterinary Research,45, 2272–2275
McKiernan, B.C. and Koritz, G.D., 1990. Plasma theophylline concentration and lung function in ponies with recurrent obstructive lung disease.Equine Veterinary Journal,22, 194–197
McKiernan, B.C., Neff Davis, C., Koritz, G.D. and Pheris, D., 1981. Pharmacokinetic studies of theophylline in dogs.Journal of Veterinary Pharmacology and Therapeutics,4, 103–110
McKiernan, B.C., Koritz, G.D., Davis, E. and Munsiff, I., 1983. Pharmacokinetic studies of theophylline in cats.Journal of Veterinary Pharmacology and Therapeutics,6, 99–104
Mitenko, P. and Ogilvie, R., 1973. Rational intravenous doses of theophylline.New England Journal of Medicine,289, 600–603
Nelder, J. and Mead, R., 1965. A simplex method for function minimization.Computers Journal,4, 300–313
Pollock, J., Kiechel, F., Cooper, D. and Weinberger, M., 1977. Relationship of serum theophylline concentration to inhibition of exercise induced bronchospasm and comparison with chromolyn.Pediatrics Springfield,60, 840–844
Rall, T.W., 1980. The xanthines. In: A.G. Gilman, L.S. Goodman and A. Gilman (eds),The Pharmacological Basis of Therapeutics, 6th edn, (McMillan, New York), 592–607
Short, C.R., Horner, M.W., Blay, P.K., Moss, M.S., Edington, N. and Clarke, C.R., 1986. The lack of effect of inoculation with equine influenza vaccine on theophylline pharmacokinetics in the horse.Journal of Veterinary Pharmacology and Therapeutics,9, 426–432
Thomson, J.R. and McPherson, E.A., 1983. Chronic obstructive pulmonary disease in the horse 2: Therapy.Equine Veterinary Journal,15, 207–210
Wade, A., 1977. InMartindale, The Extra Pharmacopoeia, 27th edn, (Pharmaceutical Press, London)
Wagner, J.G., 1983.Farmacocinética Clínica, (Reverté, Barcelona, Spain), 175–232
Yamaoka, K., Nakagawa, T. and Uno, T., 1978. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations.Journal of Pharmacokinetics and Biopharmaceutics,6, 165–175
Ziment, I., 1978. Phosphodiesterase inhibitors.Respiratory Pharmacology and Therapeutics, (W.B. Saunders, Philadelphia), 190–207
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Errecalde, J.O., Landoni, M.F. The pharmacokinetics of a slow-release theophylline preparation in horses after intravenous and oral administration. Vet Res Commun 16, 131–138 (1992). https://doi.org/10.1007/BF01839010
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01839010