Abstract
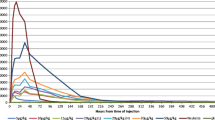
Nineteen patients with advanced cancer were entered into a phase I clinical trial of Tumor Necrosis Factor (TNF) which was designed to determine the pharmacokinetic profile, safety, and maximal tolerated dose (MTD) of the recombinant human cytokinein vivo. TNF was administered by continuous infusion for 24 hours followed by pharmacokinetics and a 120-hour infusion repeated every 3 weeks. The initial dose was 40μg/m2 and was ultimately escalated to 200μg/m2. A total of forty 5-day cycles were administered to 18 of these patients; and all were evaluable for toxicity. Toxicities in this trial included fever, chills, rigors, hypotension, headaches, seizures, lethargy, weight loss, and malaise. At all dose levels, but more significantly at the highest doses, hematological toxicities were observed and grade 3 neurotoxicity (headache and confusion), and hypotension were noted. Two patients expired during the study, and this was felt to be related to septic episodes. Because of these severe toxicities, 160μg/m2 was defined as the MTD. At 160μg/m2 peak serum levels occurred within 5–20 minutes of initiation and were not detectable 1 hour later. No anti-tumor responses were observed. No measurable plasma levels of TNF were observed with the administration of doses of 80μg/m2. This dose level could be further studied in phase II studies alone and in combination with other agents, utilizing a continuous infusion schedule.
Similar content being viewed by others
References
Adams DO, Nathan CF: Molecular mechanisms in tumor cell killing by activated macrophages. Immunology Today 4(6):166–169, 1985
Amano F, Nishijima M, Akamatsu Y: A monosaccharide precursor of escheriahia coli lipid A has the ability to induce tumor cytotoxic factor production by a murine machrophage-like cell line J774.1. J Immunol 136(11):4122–4127, 1986
Austgulen R, Espevik T, Hammerstrom J, Nissen-Meyer J: Role of monocyte cytotoxic factor in cytolysis of actinomycin D-treated WEHI 164 cells mediated by freshly isolated human adherent mononuclear blood cells. Cancer Res 46:4566–4570, 1986
Beutler B, Tkacenko V, Milsark I, Krochin N, Cerami A: Effect of gamma interferon on cachectin expression by mononuclear phagocytes: reversal of the LPS (endotoxin resistance) phenotype. J Exp Med 164:1791–1796, 1986
Bloksma N, Hofhuis FMA, Willers JMN: Role of mononuclear phagocyte function in endotoxin-induced tumor necrosis. Eur J Cancer Clin Oncol 20(3):397–403, 1984
Dinarello CA, Cannon JG, Wolf SM,et al.: Tumor necrosis factor (cachetin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med 163:1433–1450, 1986
Flick DA, Gifford GE: Comparison ofin vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Meth 68:167–175, 1984
Berman E, Rubin BY, Broxmeyer HE: Regulation of the proliferation of the established human monoblast cell line u 937, at the single cell level. Can Res 46:3309–3312, 1986
Marmenout A, Fransen L, Taverneir J, Van der Heyden J, Tizard R, Kawashima E, Shaw A, Johnson MJ, Samon D, Muller R, Ruysschaert MR, Von Vliet A, Fiero W: Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem 152(3):515–522, 1985
Mazumder A: Tumor necrosis factor (TNF) is effective and synergizes with interferon (IFN) in nude mice bearing human tumors. Proc Am Assoc Cancer Res 27:342, 1986
Green S, Dobrjansky A, Chiasson MA: Action of tumor necrosis factor (TNF) on mouse myelomonocytic leukemia (WEHI/3)in vitro andin vivo. Proc Am Assoc Cancer Res, 1981
Haranaka K, Satomi N, Sakurai A: Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and heterotransplanted human tumors in nude mice. Int J Cancer 34:263–267, 1984
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B: An endotoxin induced serum factor which causes necrosis of tumors. Proc Nat Acad Sci USA 72(9):3666–3670, 1975
Ruff MR, Gifford GE: Tumor Necrosis Factor. In: Pick E (ed) Lymphokines, Vol 2, New York Academic Press, 1981, pp 235–271
Jakubowski AA, Casper ES, Gabrilove JL,et al.: Phase I trial of intramuscularly administered tumor necrosis factor in patients with advanced cancer. J Clin Oncol 7:298–303, 1989
Sherman ML, Spriggs DR, Arthur KA,et al.: Recombinant human tumor necrosis factor administered as a five-day continuous infusion in cancer patients: phase I toxicity and effects on lipid metabolism. J Clin Oncol 6:344–350, 1988
Spriggs DR, Sherman ML, Michie H, Arthur RA, Imamura R, Wilmore D, Frai III E, Kufe DW: Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase I and pharmacologic study. J Natl Cancer Inst 80:1039–1044, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mittelman, A., Puccio, C., Gafney, E. et al. A phase I pharmacokinetic study of recombinant human tumor necrosis factor administered by a 5-day continuous infusion. Invest New Drugs 10, 183–190 (1992). https://doi.org/10.1007/BF00877244
Issue Date:
DOI: https://doi.org/10.1007/BF00877244