Abstract
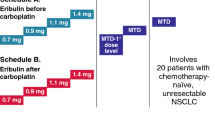
This study sought to determine the principal toxicities and feasibility of administering paclitaxel as a 3-hour infusion followed by carboplatin without and with granulocyte colony-stimulating factor (G-CSF) in chemotherapy-naive patients with stage IV non-small cell lung carcinoma (NSCLC), and to recommend doses for subsequent clinical trials. Twenty-three patients were treated with paclitaxel at doses ranging from 175 to 225 mg/m2 followed by carboplatin targeting area under the concentration-time curve (AUC) 7 or 9 mg/mL.min every 3 weeks. AUCs were targeted using the Calvert formula with estimated creatinine clearance as a surrogate for the glomerular filtration rate. A high rate of intolerable, mutually exclusive toxicities, consisting primarily of thrombocytopenia, as well as neutropenia, nausea and vomiting, and mucositis, precluded escalation of carboplatin above a targeted AUC of 7 mg/mL.min with paclitaxel 225 mg/m2, which approaches the maximum tolerated dose (MTD) of paclitaxel given as a single agent on a 3-hour schedule. Moderate to severe peripheral neurotoxicity occurred in several patients after multiple courses. Due to the heterogeneous nature of the principal toxicities and the ability to administer clinically-relevant doses of both agents in combination without G-CSF, further dose escalation using G-CSF was not performed. Nine of 23 (39%) total patients and 43% of 21 assessable patients had partial responses (PR). The recommended doses for subsequent clinical trials are paclitaxel 225 mg/m2 as a 3-hour infusion followed by carboplatin at a targeted AUC of 7 mg/mL.min. The ability to administer clinically-relevant single agent doses of paclitaxel and carboplatin in combination, as well as the significant antitumor activity noted in this phase I trial, indicate that further evaluations of this regimen in both advanced and early stage NSCLC are warranted.
Similar content being viewed by others
References
Idhe DC, Minna JD: Non-small cell lung cancer. II. Treatment. Curr Probl Cancer 15:105–54, 1991
Ganz PA, Figlin RA, Haskell CM, La Soto N, Siau J, et al.: Supportive care vs supportive care and combination chemotherapy in metastatic lung cancer. Cancer 63:1271–78, 1989
Gormier Y, Bergerson D, LaForge J, Lavandier M, Fournier M, et al.: Benefit of polychemotherapy in advanced non-small cell bronchogenic carcinoma. Cancer 50:845–9, 1982
Johnson DH, Einhorn LH: Paclitaxel plus carboplatin: An effective combination chemotherapy for advanced non-small cell lung cancer or just another Elvis siting. J Clin Oncol 13:1840–2, 1995
Grilli R, Oxman AD, Julian J: Chemotherapy for advanced non-small cell lung cancer: how much benefit is enough. J Clin Oncol 11:1866–72, 1993
Chang A, Kim K, Glick J, Anderson T, Karp D, Johnson D: Phase II study of taxol, merbarone, and piroxantrone in stage IV non-small cell lung cancer; the Eastern Cooperative Oncology Group (ECOG) results. J Natl Can Inst 85:388–94, 1993
Murphy WK, Fossella FV, Winn RJ, Shin DM, Hynes HE, et al.: Phase II study of taxol in patients with untreated non-small cell lung cancer. J Natl Can Inst 85:384–8, 1993
Israel VK, Zaretsky S, Natale RB: Phase I/II trial of combination carboplatin and Taxol in advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 13:351, 1994 (abstr)
Hainsworth JD, Greco A: Paclitaxel administered by 1-hour infusion. Cancer 74:1377–82, 1994
Hainsworth JD, Thompson DS, Greco FA: Paclitaxel by 1-hour infusion: An active drug in metastatic non-small cell lung cancer. J Clin Oncol 13:1609–14, 1995
Gatzemeir U, Pawel JY, Hencmayer M, Neuhauss R, Schluter I: Phase II study with paclitaxel in advanced inoperable non-small cell lung cancer (NSCLC) — the European experience. Lung Cancer 2:236, 1994 (suppl)
Bonomi PD, Finkelstein DM, Ruckdeschel JC, Blum RH, Green MD, et al.: Combination chemotherapy versus single agents followed by combination chemotherapy in stage IV non-small cell lung cancer: A study of the Eastern Cooperative Oncology Group. J Clin Oncol 7:1602–13, 1989
Klastersky J, Sculier JP, Lacroix H, Dabouis G, Bureau DG, et al.: A randomized study comparing cisplatin or carboplatin with etoposide in patients with advanced non-small cell lung cancer: European Organization for Research and Treatment of Cancer protocol 07861. J Clin Oncol 8:1556–62, 1990
Langer CJ, Leighton JC, Comis RL, O'Dwyer P, McAleer CA, et al.: Paclitaxel and carboplatin in combination in the treatment of advanced non-small cell lung cancer: A phase II toxicity, response, and survival analysis. J Clin Oncol 13:1860–70, 1995
Eisenhauer E, Ten Bokkel Huinink W, Swenerton KD, Gianni L, Myles J, et al.: European-Canadian randomized trial of taxol in relapsed ovarian cancer: High vs low dose and long vs. short infusion. J Clin Oncol 12:2654–66, 1994
Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SA, Donehower RC, et al.: Clinical toxicities encountered with taxol. Sem Oncol 20(Suppl 3):1–15, 1993
Schiller JH, Storer B, Tutsch K, Arzoomanian R, Alberti D, et al.: Phase I trial of 3-hour infusion of paclitaxel with or without granulocyte colony-stimulating factor. J Clin Oncol 12:241–8, 1994
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, et al.: Carboplatin dosage: Prospective evaluation of a simple formula based on renal functions. J Clin Oncol 7:1748–56, 1989
Jelliffe RW: Creatinine clearance: Bedside estimate. Ann Intern Med 79:604–5, 1973
Guidelines for Reporting of Adverse Drug Reactions. Bethesda, MD: Division of Cancer Treatment, National Cancer Institute, 1988
Foster BJ, Clagett-Carr K, Leyland-Jones B, Hoth D: Results of NCI-sponsored phase I trials with carboplatin. Cancer Treat Rev 12:43–9, 1985
Sarosy G, Kohn E, Stone DA, Rothenberg M, Jacob J, et al.: Phase I study of taxol and granulocyte colony-stimulating factor in patients with refractory ovarian cancer. J Clin Oncol 10:1165–70, 1992
Bookman MA, McGuire WP III, Kilpatrick D, Keenan E, Hogan WM, Johnson S, O'Dwyer P, Rowinsky EK, Gallion HH and Ozols RF: Carboplatin and paclitaxel in ovarian and peritoneal carcinoma: A phase I study of the Gynecologic Oncology Group. J Clin Oncol, in press 1996
Rowinsky EK, Gilbert M, McGuire WP, Ettinger DS, Forastiere A, Grochow, LB, et al.: Sequences of taxol and cisplatin: A phase I and pharmacologic study. J Clin Oncol 9:1692–1703, 1991
Georgiadis MS, Russell EK, Gazdar AF, Johnson BE: Paclitaxel cytotoxicity against human lung cancer cell lines. Clinical Cancer Res 3:449–454, 1997
Milford MJ, Bishop JF, Friedlander M, Levi JA, Goldstein D, et al.: Phase II trial of a 3-hour infusion of paclitaxel in previously untreated patients with advanced non-small cell lung cancer. J Clin Oncol 14:142–148, 1996
Peretz T, Sulkes A, Chollet P, Gelmon K, Paridaens R, et al.: A multicenter randomized study of two schedules of paclitaxel in patients with advanced breast cancer. Eur J Cancer 31A(Suppl 5):S75, 1995 (abstr).
Chatelut E, Canal P, Brunner V, Chevreau C, Pujol A, et al.: Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 87:573–80, 1995
Egorin MJ: Further refinement of carboplatin dosing. J Natl Cancer Inst 87:555–556, 1995
Jodrell DI, Egorin MJ, Canetta RM, Langenberg P, Goldbloom EP, et al.: Relationship between carboplatin exposure and tumor response and toxicity in patients with ovarian cancer. J Clin Oncol 10:520–8, 1992
LeBlanc GA, Sundseth SS, Weber GF, Waxman DJ, et al.: Platinum anticancer drugs modulate P-450 mRNA levels and differentially alter hepatic drug and steroid hormone metabolism in male and female rats. Cancer Res 5:540–547, 1992
Rowinsky EK, Citardi M, Noe DA, Donehower RC: Sequence-dependent cytotoxicity between cisplatin and the antimicrotubule agents taxol and vincristine. J Can Res Clin Oncol 119:737–743, 1993
Parker RJ, Dabholkar MD, Lee K-B, Bostoick-Burton F, Reed E: Taxol effect on cisplatin sensitivity and cisplatin cellular accumulation in human ovarian cancer cells. Monograph Natl Can Inst 15:83–88, 1993
Huizing, MT, Giaccone G, van Warmerdam LJC, Rosing H, Bakker PJM et al.: Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small cell lung cancer. J Clin Oncol 15:317–329, 1997
Gianni L, Munzone E, Capri F, Fulfaro F, Tarenzi E, et al.: Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13:2688–2699, 1995
Pronk LC, Schellens JHM, Planting AST, van den Brent MJ, Hilkens PHE, et al.: Phase I and pharmacologic study of docetaxel and cisplatin in patients with advanced solid tumors. J Clin Oncol 15:1071–1079, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rowinsky, E.K., Flood, W.A., Sartorius, S.E. et al. Phase I study of paclitaxel on a 3-hour schedule followed by carboplatin in untreated patients with stage IV non-small cell lung cancer. Invest New Drugs 15, 129–138 (1997). https://doi.org/10.1023/A:1005821125290
Issue Date:
DOI: https://doi.org/10.1023/A:1005821125290