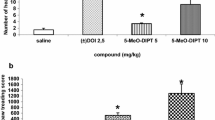
Abstract
The effects ofD,L-ϱ-chlorophenylalanine methyl ester (PCPA-methyl ester) and two of its metabolites, 2-(ϱ-chlorophenyl)-ethylamine (PCPEA) and ϱ-chlorophenylacetic acid (PCPAA), on the metabolism of serotonin (5-HT) fromD,L-5-hydroxytryptophan (5-HTP) ware studied in vitro and in vivo using the telencephalon and brainstem of the rat. For in vivo studies and some in vitro experiments, rats were injected with either 100 mg/kg PCPA-methyl ester or saline alone on days 1, 2, and 3, and were killed on day 15. When the in vivo metabolism of 5-HT was to be studied, the saline group and the PCPA group of animals were injected with 75 μg/kg [3H]D,L-5-HTP 20 min before sacrificing. With respect to the values found for the saline-injected animals, the specific activity (S.A.; dpm/nmol) of 5-HIAA was significantly greater in the telencephanol and brainstem of the animals injected with PCPA-methyl ester. The S.A. of 5-HTP was the same in both groups; the S.A. of 5-HT was lower in the telencephalon of the PCPA group than in the saline group; in the brainstem, there was no difference. In both the saline- and PCPA-injected animals, the S.A. of 5-HIAA was greater than the S.A. of 5-HT. There was no difference between the saline- and PCPA-injected animals with regard to: (1)L-5-HTP decarboxylase activity; (2)L-5-HTP-induced release of [3H]5-HT in vitro from crude nerve ending fractions (P2); or (3) in vitro uptake of [3H]D,L-5-HTP and its conversion to [3H]5-HT using the P2 fraction. In vitro studies demonstrated that the PCPEA could directly cause a large increase in the release of [3H]5-HT from the P2 fraction, whereas PCPA and PCPAA had little or no apparent effect. The data were interpreted to suggest that in the telencephalon of the animals treated with PCPA-methyl ester, there was a higher turnover of 5-HT than was found in the saline-treated group.
Similar content being viewed by others
References
Aghajanian, G.K., Rosecrans, J.A., andSheard, M.H. (1967) Serotonin: Release in the forebrain by stimulation of midbrain raphe. Science 156, 402, 403.
Anden, N.-E., Dahlstrom, A., Fuxe, K., Larsson, K., Olson L., andUngerstedt, U. (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol. Scand. 67, 313–326.
Aprison, M.H., andFischer, C.A. (1972) An attempt to estimate the free or physiologically effective pool of serotonin. Fed. Proc. Fed. Am. Soc. Exp. Biol. 31, 395.
Aprison, M.H., Hingtgen, J.N., andMcBride, W.J. (1975) Serotonergic and cholinergic mechanisms during disruption of approach and avoidance behavior. Fed. Proc. Fed. Am. Soc. Exp. Biol. 34, 1812–1822.
Boggan, W.O., Freedman, D.X., andAppel, J.B. (1973)p-Chlorophenylalanine-induced alterations in the behavioral effects of 5-hydroxytryptophan. Psychopharmacologia 33, 293–298.
Butcher, L.L., Engel, J., andFuxe, J.(1972) Behavioral, biochemical, and histochemical analyses of the central effects of monoamine precursors after peripheral decarboxylase inhibition. Brain Res. 41, 387–411.
Cavalli-Sforza, L.L., Santachiara, S.A., Wang, L., Erdelyi, E., andBarchas, J. (1974) Electrophoretic study of 5-hydroxytryptophan decarboxylase from brain and liver in several species. J. Neurochem. 23, 629–634.
Edwards, D.J., andBlau, K. (1972) The in vivo formation ofp-chloro-β-phenylethylamine in young rats injected withp-chlorophenylalanine. J. Neurochem. 19, 1829–1832.
Fischer, C.A., andAprison, M.H. (1972) Is there more than one compartment of serotonin in the central nervous system? Trans. Am. Soc. Neurochem. 3, 78.
Gál, E.M., Roggeveen, A.E., andMilard, S.A. (1970)DL-(2-14C)-p-Chlorophenylalanine as an inhibitor of tryptophan 5-hydroxylase. J. Neurochem. 17, 1221–1235.
Gray, E.G., andWhittaker, V.P. (1962) The isolation of nerve endings from brain: An electron microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. London 96, 79–88.
Jequier, E., Lovenberg, W., andSjoerdsma, A. (1967) Tryptophan hydroxylase inhibition: The mechanism by whichp-chlorophenylalanine depletes rat brain serotonin. Mol. Pharmacol. 3, 274–278.
Koe, B.K., andWeissman, A. (1966)p-Chlorophenylalanine: A specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 154, 499–516.
Korf, J., Venema, K., andPostema, F. (1974) Decarboxylation of exogenousL-5-hydroxytryptophan after destruction of the cerebral raphe system. J. Neurochem. 23, 249–252.
Lowry, O.H., Rosebrough, N.J., Farr, L.A., andRandall, R.J.(1951) Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275.
McBride, W.J., andAprison, M.H. (1973) Release of 5-hydroxytryptamine from serotonergic nerve endings by α-methyl-meta-tyramine. Pharmacol. Biochem. Behav. 1, 587–590.
McBride, W.J., Aprison, M.H., andHingtgen, J.N. (1973) Effects of α-methyl-meta-tyrosine, α-methyl-meta-tyramine and metaraminol on the serotonin content in preparations of whole tissue and synaptosomes from the telencephalon of the pigeon. Neuropharmacology 12, 769–776.
McBride, W.J., Aprison, M.H., andHingtgen, J.N. (1974) Effects of 5-hydroxytryptophan on serotonin in nerve endings. J. Neurochem. 23, 385–391.
McBride, W.J., Mahler, H.R., Moore, W.J., andWhite, F.P. (1972) Isolation and characterization of membranes from rat cerebral cortex. J. Neurobiol. 2, 73–92.
McIlwain, H., Harvey, J.A., andRodriguez, G. (1969) Tetrodotoxin on the sodium and other ions of cerebral tissues, excited electrically and with glutamate. J. Neurochem. 16, 363–370.
Padjen, A., andRandic, M. (1970) Some factors influencing the release of 5-hydroxyindol-3-ylacetic acid in the forebrain. Br. J. Pharmacol. 39, 1–18.
Shields, P.J., andEccleston, D. (1972) Effects of electrical stimulation of rat midbrain on 5-hydroxytryptamine synthesis as determined by a sensitive radioisotope method. J. Neurochem. 19, 265–272.
Shields, P.J., andEccleston, D. (1973) Evidence for the synthesis and storage of 5-hydroxytryptamine in two separate pools in the brain. J. Neurochem. 20, 881–888.
Sims, K.L., Davis, G.A., andBloom, F.E. (1973) Activities of 3,4-dihydroxy-L-phenylalanine and 5-hydroxy-L-tryptophan decarboxylases in rat brain: Assay characteristics and distribution. J. Neurochem. 20, 449–464.
Smith, J.E., Lane, J.D., Shea, P.A., McBride, W.J., andAprison, M.H. (1975) A method for concurrent measurement of picomole quantities of acetylcholine, choline, dopamine, norepinephrine, serotonin, 5-hydroxytryptophan, 5-hydroxyindoleacetic acid, tryptophan, tyrosine, glycine, aspartate, glutamate, alanine, and gamma-aminobutyric acid in single tissue samples from different areas of rat central nervous system. Anal. Biochem. 64, 149–169.
Takahashi, R., andAprison, M.H. (1964) Acetylcholine content of discrete areas of the brain obtained by a near-freezing method. J. Neurochem. 11, 887–898.
White, F.P., McBride, W.J., Mahler, H.R., andMoore, W.J. (1972) Subcellular distribution of proteins synthesized in slices of rat cerebral cortex. J. Biol. Chem. 247, 1247–1256.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McBride, W.J., Penn, P.E., Hyde, T.P. et al. Effects ofp-chlorophenylalanine on the metabolism of serotonin from 5-hydroxytryptophan. Neurochem Res 1, 437–449 (1976). https://doi.org/10.1007/BF00966235
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00966235