Abstract
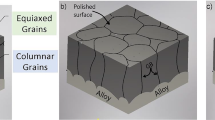
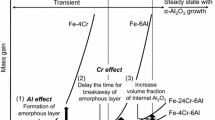
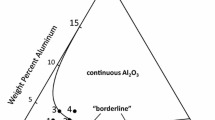
Recently a new theory was proposed to explain the effect that reactive elements have on oxide adherence. Based on data obtained on Ni-Cr-Al-Y material, this theory stated that trace quantities of sulfur in the alloy degrade adherence by weakening the metal-Al2O3 bond. The work presented here extends this concept to Fe-Cr-Al alloys by examining Al2O3 adherence on foil samples with various bulk sulfur levels obtained using high-temperature vacuum anneals. Results show that long-time vacuum anneals dramatically increase the adherence of the subsequently grown aluminum oxide, concurrent with removal of sulfur from the matrix. This evidence shows that the Al2O3-metal bond is intrinsically strong without the presence of reactive elements such as Y or rare earths in the alloy. Sulfur in the alloy, and not void formation, was found responsible for oxide spalling. In addition, voids were eliminated by reducing the sulfur concentration near the oxide-metal interface.
Similar content being viewed by others
References
D. P. Whittle and J. Stringer,Philos. Trans. R. Soc. Lond. Ser. A 295, 309–329 (1980).
H. Hindam and D. P. Whittle,Oxid. Met. 18(6), 245–285 (1982).
G. C. Wood and F. H. Stott,High Temperature Corrosion, R. A. Rapp, ed. (National Association of Corrosion Engineers, Houston, Texas, 1983), Vol. 6, pp. 227–250.
J. Stringer,Met. Rev. 11, 113–128 (1966).
J. K. Tien and F. S. Pettit,Met. Trans. 3, 1587–1599 (1972).
B. Lustman,Trans. TMS-AIME,188, 995–996 (1950).
E. J. Feiten,J. Electrochem. Soc. 108, 490–495 (1961).
C. S. Wukusick and J. F. Collins,Mat. Res. Stand. 4, 637–646 (1964).
H. Pfeiffer,Werkst. Korros. 8, 574–579 (1957).
J. E. Antill and K. A. Peakall,J. Iron Steel Inst. 205, 1136–1142 (1967).
J. M. Francis and J. A. Jutson,Corros. Sci. 8, 445–449 (1968).
F. A. Golightly, F. H. Stott, and G. C. Wood,Oxid. Met. 10(3), 163–187 (1976).
J. E. McDonald and J. G. Eberhart,Trans. TMS-AIME 233, 512–517 (1965).
J. L. Smialek and R. Browning,NASA Technical Memorandum 87168 (1985).
A. W. Funkenbusch, J. G. Smeggil, and N. S. Bornstein,Met. Trans. 16A, 1164–1166 (1985).
J. G. Smeggil, A. W. Funkenbusch, and N. S. Bornstein,Met. Trans. 17A, 923–932 (1986).
R. P. Elliott,Constitution of Binary Alloys, First Supplement (McGraw-Hill, New York, 1965), pp. 308, 575, 798, 799.
W. G. Moflatt,Handbook of Binary Phase Diagrams (General Electric Co., Corporate Research and Development Center, Schenectady, New York, 1984).
JANAF Thermochemical Tables Second Edition, D. R. Stull and H. Prophet, project directors (National Standard Reference Data Service, National Bureau of Standards, 37, June 1971).
K. C. Mills,Thermodynamic Data for Inorganic Sulphides, Selenides and Tellurides (Butterworth, London, 1974). pp. 62. 69–70. 206–209, 406–409, 677–679, 752, 794–795.
I. Barin, O. Knacke, and O. Kubaschewski,Thermochemical Properties of Inorganic Substances, Supplement (Springer-Verlag, Berlin, 1977), pp. 153, 349, 816.
R. L. Montgomery,U.S. Bureau of Mines, Reports of Investigations,5468, pp. 1–23 (1959).
M. W. Evans, InChemistry and Metallurgy of Miscellaneous Materials: Thermodynamics, L. L. Quill, ed. 1st ed. (McGraw-Hill, New York, 1950), pp. 312–320.
L. Brewer, L. A. Bromley, P. W. Gilles, and N. L. Lofgren, InChemistry and Metallurgy of Miscellaneous Materials: Thermodynamics, L. L. Quill, ed., 1st ed. (McGraw-Hill, New York, 1950), pp. 40–59.
E. G. King and W. W. Weller,U.S. Bureau of Mines, Reports of Investigations, No. 5485, pp. 1–5 (1959).
K. A. Gschneidner and N. Kippenham,Thermochemistry of the Rare Earth Carbides, Nitrides and Sulfides for Steelmaking (Rare Earth Information Center, Institute for Atomic Research, Iowa State University, Ames Iowa, 1972), pp. 18, 20.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sigler, D.R. The influence of sulfur on adherence of Al2O3 grown on Fe-Cr-Al alloys. Oxid Met 29, 23–43 (1988). https://doi.org/10.1007/BF00656348
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00656348