Abstract
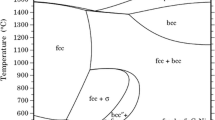
An Fe-27 w/o (weight %) Mn alloy was sulfidized at temperatures of 973, 1073, and 1173 K inflowing H2/H2S/N2 atmospheres corresponding to equilibrium sulfur pressures of 8 Pa. Steady-state parabolic kinetics were always observed after an initial period during which the instantaneous parabolic rate constant increased with time. Product scales were compact and consisted of a layer of Fe(Mn)1−x S over an inner layer of α-Mn(Fe)S. Preoxidation led to a diminution in the subsequent sulfidation rate. Conflicts between differing reports in the literature of the kinetics of this reaction are resolved, and it is concluded that the protective effect expected of an α-MnS layer is in fact possible.
Similar content being viewed by others
References
F. A. Elrefaie and W. W. Smeltzer,Oxid. Met. 16, 267 (1981).
E. M. Fryt, V. S. Bhide, W. W. Smeltzer, and J. S. Kirkaldy,J. Electrochem. Soc. 126, 683 (1979).
K. Nishida, T. Narita, T. Tani, and G. Sasaki,Oxid. Met. 14, 65 (1980).
K. Nishida and T. Narita,Proc. 8th Int. Cong. Metallic Corros. 1, 821 (1981).
M. Perez and J. P. Larpin,Oxid. Met. 24, 9 (1985).
J. P. Larpin and G. Bertrand,Reactivity of Solids 1, 75 (1985).
J. C. Colson and J. P. Larpin,Mat. Sci. Eng. 87, 11 (1987).
E. T. Turkdogan and W. L. Worrell,Trans. AIME 242, 1673 (1968).
G. Simkovich,Werkst. Korros. 21, 973 (1970).
P. D. Zelanko and G. Simkovich,Oxid. Met. 8, 343 (1974).
D. F. Wilson and O. F. Devereaux, in “Corrosionin Fossil Fuel Systems”, I. G. Wright, ed. (Electrochemical Society, Pennington, New Jersey, 1983), p. 247.
J. P. Orchard and D. J. Young,J. Electrochem. Soc. 133, 1734 (1986).
H. Buscail and J. P. Larpin,Oxid. Met. 30, 273 (1988).
D. deB. Darwent and R. Roberts, Proc.Roy. Soc. London A 216, 344 (1953).
C. D. Holland and R. G. Anthony, inFundamentals of Chemical Reaction Engineering (Prentice-Hall, Englewood Cliffs, New Jersey, 1979), p. 57.
R. A. McKee and R. E. Drushel,J. Electrochem. Soc. 130, 898 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Southwell, G., Young, D.J. Sulfidation behavior of a binary Fe-Mn alloy. Oxid Met 34, 161–172 (1990). https://doi.org/10.1007/BF00665013
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00665013