Abstract
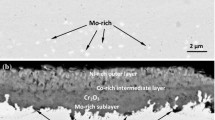
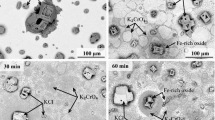
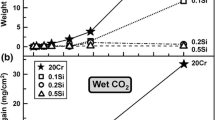
Pure nickel, Ni-20Cr and Ni-30Cr alloys wereexposed to conditions of erosion and corrosionsimultaneously at 700°C and 800°C. The exposureswere made using normal impact of an air stream loadedwith 20-μm alumina. The alumina particles flowed at therate of 400 mg per min and the velocities used were 75m/s and 125 m/s. The reaction kinetics were measureddiscontinuously by interrupting the exposure and measuring the weight loss. The specimens wereexamined using SEM-EDAX both on the surface and incross-section. Under simple oxidation, the alloyspecimens developed a thin protective layer of chromia. Under erosion-corrosion conditions, thisprotective scale was prevented from forming and thealloys were found to undergo aggressive attack at a ratethat was the same as that experienced for pure nickel, the surface oxides were identified asCr2O3 and NiO. It is proposedthat, under erosion corrosion, the erosive streamprevents the formation of a continuous layer of chromiaby removing the oxide faster than it can spread laterally. The specimen issaid to be in a state of erosion-maintained transientoxidation. This mechanism implies that it would bedifficult for protective scales to form in the presence of erosion and the oxidation behavior of analloy cannot be used as a guide to its resistance toerosion-corrosion.
Similar content being viewed by others
REFERENCES
I. Finnie, Wear 3, 87 (1960).
C. T. Kang, F. S. Pettit, and N. Birks, Met. Trans. A 18A, 1785 (1987).
S. Hogmark, A. Hammersten, and S. Soderberg, Combined Effects of Corrosion and Erosion, Proc. 6th Int. Conf. on Erosion by Liquid and Solid Impact (1983), Paper 37.
D. M. Rishel, F. S. Pettit, and N. Birks, Mater. Sci. Engin. A143, 197 (1991).
I. G. Wright, V. Nagarajan, and J. Stringer, Oxid. Met. 25, 175 (1986).
M. M. Stack, F. H. Stott, and G. C. Wood, Corros. Sci. 33, 965 (1992).
C. Wagner, Z. Electrochem. 63, 772 (1959).
G. H. Meier and R. A. Rapp, Z. Phys. Chem. 74, 168 (1971).
T. Hodgkins, G. C. Wood, D. P. Whittle, and B. D. Bastow, Oxid. Met. 12, 439 (1978).
V. K. Sethi and I. G. Wright, A description of erosion-oxidation based on scale removal and scale erosion, in Proc. Int. Conf. Corrosion- Erosion- Wear of Materials at Elevated Temperatures, A. V. Levy, ed. (Berkeley, CA, 1991), p. 18–1.
I. G. Wright, V. K. Sethi, and V. Nagarajan, Trans. ASME 113, 616 (1991).
G. Sundararajan, Solid particle erosion of metallic materials at elevated temperatures, Proc. Int. Conf. Corrosion- Erosion- Wear of Materials at Elevated Temperatures, A. V. Levy, ed. (Berkeley, CA, 1991), p. 11–1.
G. Sundararajan, Wear of Materials (ASME Orlando, FL, 1991), p. 111.
C. T. Kang, F. S. Pettit, and N. Birks, Met. Trans. A18, 1765 (1987).
N. Birks, F. S. Pettit, and B. Peterson, The behavior of oxide scales on metals under erosion- corrosion at high temperatures in 4th International Colloquium on the Corrosion and Protection of Materials at High Temperature,R. Krutenak and R. Streiff, eds. (Les Embiez, France, May 20-24, 1996).
A. V. Levy, Solid Particle Erosion and Erosion- Corrosion of Materials (ASM Int. Materials Park, OH, 1995), p. 105.
Rights and permissions
About this article
Cite this article
Link, R.J., Birks, N., Pettit, F.S. et al. The Response of Alloys to Erosion-Corrosion at High Temperatures. Oxidation of Metals 49, 213–236 (1998). https://doi.org/10.1023/A:1018886509459
Issue Date:
DOI: https://doi.org/10.1023/A:1018886509459