Abstract
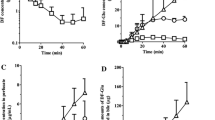
The distribution kinetics of salicylic acid in the single-pass isolated perfused rat liver has been investigated under varying conditions of perfusate flow (15 to 30 ml min−1) and of salicylate perfusate concentration (0, 100, 200 mg 1−1) using statistical moment analysis and the two-compartment axial dispersion model. Salicylic acid was not metabolised during the experiment. The perfusate did not contain binding protein. As flow rate was increased, the maximum fraction output per second (f(t)max) increased and the mean transit time (MTTH) decreased, while tmax became shorter for both tritiated water and 14C-salicylic acid. Increasing the salicylate perfusate concentration profoundly affected the frequency outflow profile of 14C-salicylic acid, but not that of tritiated water. The one-compartment axial dispersion model adequately described the frequency outflow profile for tritiated water, whereas the two-compartment form, which incorporates a cellular permeability barrier, provided a better description of the 14C-salicylic acid outflow data. The estimated two-compartment axial dispersion model parameters for 14C-salicylic acid, DN, the dispersion number (0.08 ± 0.03), k12, the influx rate constant (0.56 ± 0.04 sec−1) and k21, the efflux rate constant (0.095 ± 0.01 sec−1) were independent of perfusate flow rate. The in situ permeability-surface area product for 14C-salicylic acid (4.6 ± 0.7 ml min−1g−1 liver) was in good agreement with literature estimates obtained from in vitro hepatocyte experiments, suggesting that the permeability barrier is at the hepatocyte membrane. Whereas DN and k12 were uninfluenced by, k21 displayed a positive correlation with, salicylate perfusate concentration. This correlation was most likely due to decreased intracellular salicylate binding.
Similar content being viewed by others
REFERENCES
G. R. Wilkinson. Clearance approaches in pharmacology. Pharmacol. Rev. 39:1–45 (1987).
M. Rowland, D. Leitch, G. Fleming, B. Smith. Protein binding and hepatic clearance: discrimination between models of hepatic clearance with diazepam, a drug of high intrinsic clearance, in the isolated perfused rat liver preparation. J. Pharmacokinet. Biopharm. 12:129–147 (1984).
A. M. Evans, Z. Hussein, M. Rowland. A two-compartment dispersion model describes the hepatic outflow profile of diclofenac in the presence of its binding protein. J. Pharm. Pharmacol. 43:709–714 (1991).
J. M. Diaz-Garcia, A. M. Evans, M. Rowland. Application of the axial dispersion model of hepatic drug elimination to the kinetics of diazepam in the isolated perfused rat liver. J. Pharmacokinet. Biopharm. 20:171–193 (1992).
A. M. Evans, Z. Hussein, M. Rowland. Influence of albumin on the distribution and elimination of diclofenac in the isolated perfused rat liver: Analysis by impulse-response technique and the dispersion model. J. Pharm. Sci. 82:421–428 (1993).
M. Rowland, A. M. Evans. Physiologic models of hepatic elimination. New Trends in Pharmacokinetics. Eds A. Rescigno, A. K. Thakur. Plenum Press, New York, 1991.
M. S. Roberts, M. Rowland. A dispersion model of hepatic elimination. 1. Formulation of the model and bolus considerations. J. Pharmacokinet. Biopharm. 14:227–260 (1986).
Z. Hussein, A. M. Evans, M. Rowland. Physiologic models of hepatic clearance: Influence of altered protein binding on the elimination of diclofenac in the isolated perfused rat liver. J. Pharm. Sci. 82:880–885 (1993).
Y. Yano, K. Yamaoka, Y. Aoyama, H. Tanaka. Two-compartment dispersion model for analysis of organ perfusion system of drugs by fast inverse laplace transform (FILT). J. Pharmacokinet. Biopharm. 17:179–202 (1989).
E. Nelson, M. Hanano, G. Levy. Comparative pharmacokinetics of salicylate elimination in man and rats. J. Pharmacol. Exp. Ther. 153:159–166, 1966
T. Yoshikawa, Y. Sugiyama, Y. Sawada, T. Iga, M. Hanano. Effect of pregnancy on tissue distribution of salicylate in rats. Drug Metab. Dispos. 12:500–505 (1984).
J. Hirate, Y. Kato, I. Horikoshi, S. Nagase, C. T. Ueda. Further observations on the disposition characteristics of salicylic acid in analbuminemic rats. Biopharm. Drug Dispos. 10:299–309 (1989).
S. H. Dromgoole, D. E. Furst. Salicylates. In: Applied Pharmacokinetics Eds: W. E. Evans, J. J. Schentag, W. J. Jusko. Applied Therapeutic Inc., Spokane, WA, 1986.
M. Ichikawa, S. C. Tsao, T-H. Lin, S. Miyauchi, Y. Sawada, T. Iga, M. Hanano, Y. Sugiyama. Albumin-mediated transport phenomenon observed for ligands with high permeability: Effect of unstirred water layer in the disse's space of the rat liver. Hepatol. 16:38–49 (1992).
B. E. Cham, D. Johns, F. Bochner, D. M. Imhoff, M. Rowland. Simultaneous liquid-chromatographic separation of salicylic acid, salicyluric acid and gentisic acid in plasma. Clin. Chem. 25:1420–1425, (1979).
H. G. Boxenbaum, S. Riegelman, R. M. Elashoff. Statistical estimations in pharmacokinetics. J. Pharmacokinet. Biopharm. 2:123–148 (1974).
Y. Yano, K. Yamaoka, H. Yasui, T. Nakagawa. Effect of perfusion rate on the local disposition of cefixime in liver perfusion system based on two-compartment dispersion model. Drug Metab. Dispos. 19:1022–1027 (1991).
C. A. Goresky. Kinetic interpretation of hepatic multiple-indicator dilution studies. Am. J. Physiol. 245:G1–G12 (1983).
M. S. Roberts, S. Fraser, A. Wagner, L. McLeod. Residence time distribution of solutes in the perfused rat liver using a dispersion model of hepatic elimination: 1. Effect of changes in perfusate flow rate and albumin concentration on sucrose and taurocholate. J. Pharmacokinet. Biopharm. 18:209–234 (1990).
C. A. Goresky, M. Silverman. Effect of correction of catheter distortion on calculated liver sinusoidal volumes. Am J. Physiol. 207:883 (1964).
C-H. Chou, A. M. Evans, G. Fornasini, M. Rowland. Relationship between lipophilicity and hepatic dispersion and distribution for a homologous series of barbiturates in the isolated perfused in situ rat liver. Drug Metab. Dispos. 21:933–938 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussein, Z., McLachlan, A.J. & Rowland, M. Distribution Kinetics of Salicylic Acid in the Isolated Perfused Rat Liver Assessed Using Moment Analysis and the Two-Compartment Axial Dispersion Model. Pharm Res 11, 1337–1345 (1994). https://doi.org/10.1023/A:1018958915171
Issue Date:
DOI: https://doi.org/10.1023/A:1018958915171