Abstract
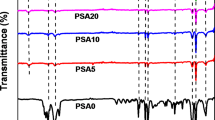
Nylon 610 is a hydrophilic polymer with considerable potential as a membrane for drug microencapsulation. To better understand drug transport through such membranes, the influence of the solvents and monomers used in the synthesis of nylon films were examined using a full factorial study. Nylon 610 films were synthesized by an interfacial polycondensation reaction using hexamethylenediamine (HD) in the water phase and sebacoyl chloride (SC) in the organic phase, which was a solvent blend of chloroform and trichlorotrifluoroethane at ratios of 1:1, 1:4, and 4:1. Monomer concentrations studied were 0.2, 0.4, and 0.6 M with respect to their appropriate phase, while the monomer ratios were 1:1, 3:1, and 1:3. The molecular weight, porosity, thickness, and crystallinity of the films were characterized. The transport of potassium chloride, hydrocortisone, and m-cresol was studied at 25°C as a function of the syntheses variables. Potassium chloride was selected to measure the porosity of the membrane. Hydrocortisone and m-cresol, a known solvent for nylon 610, were used to study pore and solution-diffusion transport, respectively. The molecular weight of the films was proportional to the chloroform concentration. As the molecular weight increased, film thickness, porosity, and hydrocortisone permeability increased. As the molecular weight decreased, film thickness and porosity decreased, while m-cresol permeability increased. These results can be explained on the basis of HD ability to readily partition into a good solvent such as chloroform permitting high molecular weight polymer to form before precipitation.
Similar content being viewed by others
REFERENCES
R. Arshady, Microspheres and Microcapsules: A Survey of Manufacturing Techniques. Part 1: Suspension Cross-Linking. Polymer Engineering and Science 29:1746 (1989).
P. W. Morgan, and S. L. Kwolek. Interfacial polycondensation. II. Fundamentals of polymer formation at liquid interfaces. Journal of Polymer Science 40:299–327 (1959).
J. W. McGinity, and G. W. Cuff. Nylon-encapsulated pharmaceuticals. Methods in Enzymology 112:87–101 (1985).
J. Brandup, and E. H. Immergut. Polymer Handbook, 3rd ed., New York: John Wiley & Sons, 1989.
P. W. Atkins. Physical Chemistry, New York: W. H. Freeman and Company, 1986.
E. A. Turi. Thermal Characterization of Polymer Materials, Academic Press, New York, 1981, pp. 235–354.
R. C. Weast. Handbook of Chemistry and Physics, 52nd ed., Cleveland, OH: The Chemical Rubber Co., 1972.
P. J. M. Stout, N. Khoury, J. Mauger, and S. Howard. Evaluation of a tube method for determining diffusion coefficients for sparingly soluble drugs. Journal of Pharmaceutical Sciences 75:65 (1986).
C. R. Wilke, and P. Chang. AIChE J. 1:264–270 (1955).
E. Mathiowitz, and M. D. Cohen. Polyamide microcapsules for controlled release. I. Characterization of the membranes. Journal of Membrane Science 40:1–26 (1989c).
L. Madelkern, and A. L. Allan. Effect of molecular weight on crystallization melting and morphology of long chain molecules. Journal of Polymer Science 4:447 (1966).
H. E. Bair, R. Salovey, and T. W. Huseby. Melting and annealing of polyethylene single crystals. Polymer 8:9 (1967).
P. J. Holdsworth, and A. Turner-Jones. Melting behavior of heat crystallized poly(ethylene terephthalate). Polymer 12:195 (1971).
G. E. Sweet, and J. P. Bell. Multiple endotherm melting behavior in relation to polymer morphology. Journal of Polymer Science, Polymer Physics Edition 10:1273 (1972).
D. Poncelet, B. Poncelet De Smet, and R. J. Neurfeld. Nylon membrane formation in biocatalyst microencapsulation: physicochemical modeling. Journal of Membrane Science 50:249–267 (1990).
L. J. J. M. Janssen, and K. te Nijenhuis. Encapsulation by interfacial polycondensation. I. The capsule production and a model for wall growth. Journal of Membrane Science 65:59–68 (1992).
P. W. Morgan. Condensation Polymers by Interfacial and Solution Methods, New York: Interscience, 1965.
H. J. Friendlander. Encyclopedia of Polymer Science and Technology, Vol. 8. New York: John Wiley Sons, 1965.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phares, K., Cho, M., Johnson, K. et al. Drug Transport Across Nylon 610 Films: Influence of Synthesis Variables. Pharm Res 12, 248–256 (1995). https://doi.org/10.1023/A:1016235111210
Issue Date:
DOI: https://doi.org/10.1023/A:1016235111210