Abstract
Purpose. The alteration of the pharmacokinetic parameters of the poly-peptide BBI through conjugation with palmitic acid was examined.
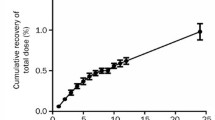
Methods. 125I-BBI or l25I-Pal-BBI was administered iv to 6 week old CF-1 mice at a dose of 3mg/kg. The mice were sacrificed at 5, 10, 20, 60, 120, 240, 360, and 480 min and the total radioactivity was determined for blood and each organ. The blood was analyzed on a Sephadex G-50 size-exclusion column to determine the amount of intact polypeptide present in the blood. From the amount of intact polypeptide at each time point, the pharmacokinetic parameters were determined.
Results. By conjugating three palmitic acids to each BBI molecule, the area under the curve (AUC) and mean residence time (MRT) increase by a factor of 10.8 and 2.8, respectively. There was also a difference in the organ distribution between the two treatments; while 125I-BBI was rapidly cleared from the kidneys, l25I-Pal-BBI was predominantly to the liver. Subsequent studies suggested that the binding of the conjugate to non-albumin serum proteins was most likely the cause of the altered pharmacokinetics.
Conclusions. The residence time in the blood and the lipophilicity of BBI were increased upon conjugation with palmitic acid through a reversible disulfide linkage. Pharmacokinetic studies showed an increase in the AUC and a decrease in kidney clearance in palmitic acid conjugates, indicating a potential increase of the therapeutic efficacy of the polypeptide drug.
Similar content being viewed by others
REFERENCES
S. Muranishi, A. Sakai, K. Yamada, M. Murakami, K. Takada and Y. Kiso. Lipophilic peptides: synthesis of lauroyl thyrotropin-releasing hormone and its biological activity. Pharm. Res. 8:649–652 (1991).
E. Yodoya, K. Uemura, T. Tenma, T. Fujita, M. Murakami, A. Yamamoto and S. Muranishi. Enhanced permeation of tetragastrin across the rat intestinal membrane and its reduced degradation by acylation with various fatty acids. J. Pharmacol. Exp. Therap. 271:1509–1513 (1994).
M. Hashizume, T. Douen, M. Murakami, A. Yamamoto, K. Takada and S. Muranishi. Improvement of large intestinal absorption of insulin by chemical modification with palmitic acid in rats. J. Pharm. and Pharmacol. 44:555–559 (1992).
K. Y. Hostetler, D. D. Richman, E. A. Forssen, L. Selk, R. Basava, M. F. Gardner, S. Parker, and C. Basava. Phospholipid prodrug inhibitors of the HIV protease. Biochem. Pharmacol. 48:1399–1404 (1994).
D. E. Bowman. Fractions derived from soy beans and navy beans which retard tryptic digestion of casein. Proc. Soc. Exp. Biol. Med. 57:139–140 (1944).
A. R. Kennedy. Protease inhibitors as cancer chemopreventive agents, Plenum Press, New York, 1993.
H. P. Witschi and A. R. Kennedy. Modulation of lung tumor development in mice with the soybean-derived Bowman-Birk protease inhibitor. Carcinogenesis, 10:2275–2277 (1989).
V. P. Torchilin, V. G. Omel'yaneko, A. L. Kilbanov, A. L. Mikhailov, V. L. Gol'danskii and V. N. Smirnov. Incorporation of hydrophilic protein modified with hydrophobic agent into liposome membrane. Biochim. Biophys. Acta. 602:511–521 (1980).
F. Al-Obeido, V. J. Hruby, N. Yaghoubi, M. M. Marwan and M. E. Hadley. Synthesis and biological activities of fatty acid conjugates of a cyclic lactam alpha-melanotropin. J. Med. Chem. 35:118–123 (1992).
H. M. Ekrami, A. R. Kennedy and W. C. Shen. Water-soluble fatty acid derivatives as acylating agents for reversible lipidization of polypeptides. FEBS Lett. 371:283–286 (1995).
P. C. McConahey and F. J. Dixon. Radiation of proteins by the use of the chloramine-T method. Methods Enzymol. 70:221–247 (1980).
B. Davies and T. Morris. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093–1095 (1993).
W. C. Shen, L. R. Honeycutt, H. Ekrami and J. Wang. Pharmacokinetics and Biodistribution of Pal-BBI, a fatty acid-polypeptide conjugate. Proc. Internat. Symp. Control. Rel. Bioact. Mater., 887–888 (1996).
I. P. Gladysheva, O. V. Polekhina, W. C. Shen, A. A. Shevchenko, N. F. Kazanskaya and N. I. Larionva. Structure and biological properties of Bowman-Birk soybean proteinase inhibitor conjugate with block copolymer of ethylene oxide and propylene oxide. Biochem. (Moscow). 60:385–391 (1995).
J. Wan, S. Persiani and W. C. Shen. Transcellular processing of disulfide-and thioether-linked peroxidase-polylysine conjugates in cultured MDCK epithelial cells. J. Cell. Physiol. 145:9–15 (1990).
M. Taub, J. Wan and W. C. Shen. Transepithelial transport of tyramine across filter-grown MDCK cells via a poly (D-lysine) carrier. Pharm. Res. 11:1250–1256 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Honeycutt, L., Wang, J., Ekrami, H. et al. Comparison of Pharmacokinetic Parameters of a Polypeptide, the Bowman-Birk Protease Inhibitor (BBI), and Its Palmitic Acid Conjugate. Pharm Res 13, 1373–1377 (1996). https://doi.org/10.1023/A:1016078118033
Issue Date:
DOI: https://doi.org/10.1023/A:1016078118033