Abstract
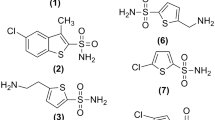
Four new aromatic sulfonamides were synthesized and purified by standard techniques. Two were unsubstituted, primary sulfonamides and two possessed substituents on the sulfonamide nitrogen. The affinity of the inhibitors for the enzyme carbonic anhydrase was determined in terms of the inhibitory potency, which was found to be dependent on the presence of an unsubstituted sulfonamide group. Binding studies were performed in erythrocyte suspensions using a range of concentrations and the unbound, extracellular concentrations were determined by high-performance liquid chromatographic (HPLC) assay. The dissociation constant of binding and the total binding capacity of the erythrocytes were estimated by nonlinear regression using a two-site binding model. The affinity of the compounds for erythrocytes reflected their inhibitory potency against the enzyme. Binding to plasma proteins was more dependent on lipophilicity and pK a and was stronger for the substituted sulfonamides. Pharmacokinetic studies in rats showed that the unsubstituted sulfonamides with a high affinity for carbonic anhydrase in erythrocytes have longer half-lives and lower clearance values than the substituted sulfonamides which were more strongly bound to plasma proteins. However, comparison of unbound clearance values showed that the variations in molecular structure, which produced differences in carbonic anhydrase binding and in distribution, also produced variations in susceptibility to elimination processes.
Similar content being viewed by others
REFERENCES
M. Gibaldi, G. Levy, and P. J. McNamara. Clin. Pharmacol. Ther. 24:1–8 (1978).
J. J. Coffey, F. J. Bullock, and P. T. Schoenemann. J. Pharm. Sci. 60:1623–1628 (1973).
J. Jansen. J. Pharmacokin. Biopharm. 9:15–26 (1981).
T. Fujita. J. Med. Chem. 15:1049–1056 (1977).
J. K. Seydel. In J. C. Dearden (ed.), Quantitative Approaches to Drug Design, Elsevier, Amsterdam, 1983, pp. 163–181.
T. H. Maren. Physiol. Rev. 47:595–781 (1967).
P. J. Wistrand and T. Wåhlstrand. Biochim. Biophys. Acta 481:71–72 (1977).
T. Wåhlstrand, K.-G. Knuutila, and P. J. Wistrand. Scand. J. Clin. Lab. Invest. 39:503–509 (1979).
H. Krebbs. Biochemistry 43:525–528 (1948).
A. Vedani and E. F. Mayer. J. Pharm. Sci. 73:352–358 (1984).
N. Kakeya, N. Yata, A. Kamade, and M. Aoki. Chem. Pharm. Bull. 17:2000–2007 (1975).
C. Hansch, J. McClarin, T. Klein, and R. Lanridge. Mol. Pharmacol. 27:493–498 (1986).
B. A. Mulley, G. D. Parr, and R. M. Rye. Eur. J. Clin. Pharmacol. 17:203–207 (1980).
B. Beerman, K. Hellstrom, B. Lindstrom, and A. Rosen. Clin. Pharmacol. Ther. 17:424–432 (1975).
J. T. Lettieri and S. T. Portelli. J. Pharmacol Exp. Ther. 224:269–272 (1983).
L. Aarons, S. Toon, and M. Rowland. J. Pharmacol. Meth. 12:337–346 (1986).
A. M. Islam, I. B. Hammout, and M. M. Atwa. Egypt. J. Chem. 17:689–697 (1974).
C. Alsen and F. K. Ohnesorge. Zeitschr. Klin. Chem. Klin. Biochem. 11:329–332 (1974).
J. E. A. McIntosh. Biochem. J. 114:463–467 (1969).
J. E. Coleman. Pharmacol. Rev. 15:221–242 (1975).
S. Toon. Ph.D. thesis, University of Manchester, Manchester, U.K., 1981.
S. Toon and M. Rowland. J. Pharmacol. Meth. 5:321–323 (1981).
P. J. Wistrand. Acta Physiol. Scand. 113:417–426 (1981).
T. H. Maren and E. O. Couto. Arch. Biochem. Biophys. 196:501–510 (1979).
W. F. Bayne, L.-C. Chu, and S. Theeuwe. J. Pharm. Sci. 68:912–913 (1979).
G. Lonnerholm, P. J. Wistrand, and E. Baraney. Acta Physiol. Scand. 126:51–60 (1986).
M. Gibaldi and D. Perrier. Pharmacokinetics, 2nd ed., Marcel Dekker, New York, 1982.
M. K. Cassidy and J. B. Houston. Drug Metab. Dispos. 12:619–624 (1980).
G. Beisenherz, F. W. Koss, L. Klatt, and B. Binder. Arch. Int. Pharmacodyn. 166:76–93 (1966).
F. Eicholtz and I. Iravani. Arzneim. Forsch. 15:538–542 (1965).
H. Weber, W. Aumuller, R. Weyer, K. Muth, and F. H. Schmidt. U.S. patent 3426067 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boddy, A., Edwards, P. & Rowland, M. Binding of Sulfonamides to Carbonic Anhydrase: Influence on Distribution Within Blood and on Pharmacokinetics. Pharm Res 6, 203–209 (1989). https://doi.org/10.1023/A:1015957315462
Issue Date:
DOI: https://doi.org/10.1023/A:1015957315462