Abstract
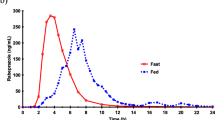
The absorption characteristics of ranitidine after delivery to three locations in the gastrointestinal tract were compared in an open-label study of eight healthy males. Subjects received ranitidine HCl (150 mg) for injection via a nasoenteric tube directly into their stomach, jejunum, or cecum sequentially in three separate periods (24 hr apart). Plasma samples were collected at periodic time intervals for 12 hr following each dosing and analyzed for ranitidine concentration by high-pressure liquid chromatography. Mean concentrations following cecal dosing were lower (P < 0.05) than concentrations following gastric or jejunal dosing at each sampling time except baseline. Mean concentrations following gastric and jejunal dosing were similar except at 2 hr (gastric > jejunal). Mean pharmacokinetic parameters for cecal administration were different (P < 0.05) from either the gastric or the jejunal periods with the exception of Tmax. There was no difference in any pharmacokinetic parameter after gastric or jejunal dosing. The relative bioavailability after cecal administration was less than 15% of that observed after administration into the stomach or jejunum. Additionally, Wagner-Nelson analysis indicated that the rate of ranitidine absorption was much slower following cecal administration than after gastric or jejunal dosing. Two plasma concentration peaks were observed in three of eight subjects after gastric dosing, in eight of eight subjects after jejunal dosing, and in zero of eight subjects after cecal dosing. These data demonstrate that the absorption profile of ranitidine is equivalent, in extent and duration, after delivery to the stomach or jejunum, while absorption from the cecum is significantly less. In addition, the two plasma concentration peaks commonly seen with ranitidine administration are not secondary to variations in gastric emptying as has been hypothesized.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Williams, M.F., Dukes, G.E., Heizer, W. et al. Influence of Gastrointestinal Site of Drug Delivery on the Absorption Characteristics of Ranitidine. Pharm Res 9, 1190–1194 (1992). https://doi.org/10.1023/A:1015860007380
Issue Date:
DOI: https://doi.org/10.1023/A:1015860007380