Abstract
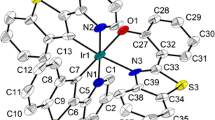
The energetics of the oxidative additive of I2 to [Ir(Μ-L)(CO)2]2 [L =t-buthylthiolate (StBu), 3,5-dimethylpyrazolate (3,5-Me2pz), and 7-azaindolate (7-aza)] complexes was investigated by using the results of reaction-solution calorimetric measurements, X-ray structure determinations, and extended Hückel (EH) molecular orbital calculations. The addition of 1 mol of iodine to 1 mol of [Ir(Μ-L)(CO)2]2, in toluene, leads to [Ir(Μ-L)(I)(CO)2]2, with the formation of two Ir-I bonds and one Ir-Ir bond. The following enthalpies of reaction were obtained for this process: −125.8±4.9 kJ mol−1 (L = StBu), −152.0±3.8 kJ mol−1 (L=3,5-Me2pz), and −205.9±9.9 kJ mol¹ (L=7-aza). These results are consistent with a possible decrease of the strain associated with the formation of three-, four-, and five-membered rings, respectively, in the corresponding products, as suggested by the results of EH calculations. The calculations also indicate a slightly stronger Ir-Ir bond for L = 3,5-Me2pz than for L= StBu despite the fact that the Ir-Ir bond lengths are identical for both complexes. The reaction of 1 mol of [Ir(Μ-StBu)(CO)2]2 with 2 mol of iodine to yield [Ir(Μ-StBu)(I)2(CO)2]2 was also studied. In this process four Ir-I bonds are formed, and from the corresponding enthalpy of reaction (−186.4±2.7 kJ mol−1) a solution phase Ir-I mean bond dissociation enthalpy in [Ir(Μ-StBu)(I)2(CO)2]2,\(\overline {DH} _{\sin } (Ir - I) = 122.2 \pm 0.7 kJ mol^{ - 1} \), was derived. This value is lower than most\(\overline {DH} _{\sin } (Ir - I)\) values reported for octahedral mononuclear Ir111 complexes. New large-scale syntheses of the [Ir(Μ-L)(CO)2]2 complexes, with yields up to 90%, using [Ir(acac)(CO)2] as starting material, are also reported. The X-ray structures of [Ir(Μ-L)(I)(CO)2]2 (L=StBu and 3,5-Me2pz) complexes have been determined. For L=StBu the crystals are monoclinic, space group P2l/c,a=10.741(2) å,b= 11.282(3) å,c=18.308(3) å,Β=96.71(1)‡, andZ=4. Crystals of theΜ-3,5-Me2pz derivative are monoclinic, space group P2l/n,a=14.002(3) å,b= 10.686(1) å,c=15.627(3) å,Β=112.406(8)‡, andZ=4. In both complexes the overall structure can be described as two square-planar pyramids, one around each iridium atom, with the iodine atoms in the apical positions, and the equatorial positions occupied by two CO groups and the two sulfur atoms of the StBu ligands, or two N atoms of the pyrazolyl ligands. In the case of L=StBu the pyramids share a common edge defined by the two bridging sulfur atoms and for L =3,5-Me2pz they are connected through the two N-N bonds of the pyrazolyl ligands. The complexes exhibit short Ir-Ir single bonds of 2.638(1) å for L=StBu and 2.637(1) å for L=3,5-Me2Pz. The oxidative addition of iodine to [Ir(Μ-3,5-Me2pz)(CO)2]2 results in a remarkable compression of 0.608 å in the Ir-Ir separation.
Similar content being viewed by others
References
For a recent review and leading references see Atwood, J. D. InComprehensive Organometallic Chemistry II; Abel, E. W.; Stone, F. G. A.; Wilkinson, G., Eds.; Elsevier: Oxford, 1995; Vol. 8; pp. 324–332.
He, X.; Maisonnant, A.; Dahan, F.; Poilblanc, R.Organometallics 1991,10, 2443.
Ciriano, M. A.; Pérez-Torrente, J.; Oro, L. A.J. Organomet. Chem. 1993,445, 273.
Ciriano, M. A.; Viguri, F.; Oro, L. A.; Tiripicchio, A.; Tiripicchio-Camellini, M.Angew. Chem. Int. Ed. Engl. 1987,26, 444.
Ciriano, M. A.; Sebastián, S.; Oro, L. A.; Tiripicchio, A.; Tiripicchio-Camellini, M.; Lahoz, F. J.Angew. Chem. Int. Ed. Engl. 1988,26, 402.
Pinillos, M. T.; Elduque, A.; Oro, L. A.; Lahoz, F. J.; Bonati, F.; Tiripicchio, A.; Tiripicchio-Camellini, M.J. Chem. Soc. Dalton Trans. 1990, 989.
Fernández, M. J.; Modrego, J.; Lahoz, F. J.; López, J. A.; Oro, A.J. Chem. Soc. Dalton Trans. 1990, 2587.
Ciriano, M. A.; Pérez-Torrente, J.; Oro, L. A.J. Organomet. Chem. 1993,445, 267.
Bonnet, J.-J.; Kalck, P.; Poilblanc, R.Angew. Chem. Int. Ed. Engl. 1980,19, 551.
Kalck, P.; Bonnet, J.-J.Organometallics 1982,1, 1211.
Beveridge, K. A.; Bushnell, G. W.; Dixon, K. R.; Eadie, D. T.; Stobart, S. R.; Atwood, J. L.; Zaworotko, M. J.J. Am. Chem. Soc. 1982,104, 920.
Coleman, A. W.; Eadie, D. T.; Stobart, S. R.; Atwood, J. L.; Zaworotko, M. J.J. Am. Chem. Soc. 1982,104, 922.
Beveridge, K. A.; Bushnell, G. W.; Stobart, S. R.; Atwood, J. L.; Zaworotko, M. J.Organometallics 1983,2, 1447.
Atwood, J. L.; Beveridge, K. A.; Bushnell, G. W.; Dixon, K. R.; Eadie, D. T.; Stobart, S. R.; Zaworotko, M.J. Inorg. Chem. 1984,23, 4050.
Harrison, D. G.; Stobart, S. R.J. Chem. Soc., Chem. Commun. 1986, 285.
Brost, R. D.; Fjeldsted, D. O. K.; Stobart, S. R.J. Chem. Soc., Chem. Commun. 1989, 488.
Brost, R. D.; Stobart, S. R.J. Chem. Soc., Chem. Commun. 1989, 498.
Brost, R. D.; Stobart, S. R.Inorg. Chem. 1989,28, 4309.
Cotton, F. A.; Lahuerta, P.; Sanaú, M.; Solana, I.; Schwotzer, W.Inorg. Chem. 1988,27, 2131.
Caspar, J. V.; Gray, H. B.J. Am. Chem. Soc. 1984,106, 3029.
Rodman, G. S.; Daws, C. A.; Mann, K. R.Inorg. Chem. 1988,27, 3347.
Pitt, C. G.; Monteith, L. K.; Ballard, L. F.; Collman, J. P.; Morrow, J. C.; Roper, W. R.; Ulk, D.J. Am. Chem. Soc. 1966,88, 4286.
Bonati, F.; Ugi, R.Chem. Ind. (Rome) 1964,46, 1332.
Nussbaum, S.; Rettig, S. J.; Storr, A.; Trotter, J.Can. J. Chem. 1985,63, 692.
De Mountauzon, D.; Poilblanc, R.Inorg. Synth. 1980,20, 236.
Sheldrick, G. M. InCrystallographic Computing 3; Sheldrick, G. M.; Krüger, C.; Goddard, R., Eds.; Oxford University Press: Oxford, 1985.
Sheldrick, G. M. University of Göttingen, 1985.
Johnson, C. K.ORTEP II, A Fortran Thermal-ellipsoid Plot Program for Crystal Structure Illustrations; Report ORNL-5138, Oak Ridge National Laboratory: Oak Ridge, TN, 1976.
Keller, E.SCHAKAL92, University of Freiburg, Germany, 1992.
International Tables for X-Ray Crystallography; Kynoch Press: Birmingham, 1974; Vol. 4.
Calhorda, M. J.; Carrondo, M. A. A. F. de C. T.; Dias, A. R.; GalvÃo, A. M.; Garcia, M. H.; Martins, A. M.; Minas da Piedade, M. E.; Pinheiro, C. I.; RomÃo, C. C.; Martinho Simões, J. A.; Veiros, L. F.Organometallics 1991,10, 483, and references citded therein.
Leal, J. P.; Pires de Matos, A.; Martinho Simões, J. A.J. Organomet. Chem. 1991,403, 1.
Hoffmann, R.J. Chem. Phys. 1963,39, 1397.
Hoffmann, R.; Lipscomb, W. N.J. Chem. Phys. 1962,36, 2179.
Ammeter, J. H.; Bürgi, H.-B.; Thibeault, J. C.; Hoffmann, R.J. Am. Chem. Soc. 1978,100, 3686.
Mealli, C.; Proserpio, D. M.J. Chem. Ed. 1990,67, 39.
Allen, F. H.; Davies, J. E.; Galloy, J. J.; Johnson, O.; Kennard, O.; Macrae, C. F.; Watson, D. G.Cambridge Structural Database, J. Chem. Inf. Comput. Sci. 1991,31, 204.
Amane, M. E.; Maisonnat, A.; Dahan, F.; Poiblanc, R.New J. Chem. 1988,12, 661.
Maisonnat, A.; Bonnet, J.-J.; Poiblanc, R.Inorg. Chem. 1980,19, 3168.
Megehee, E. G.; Johnson, C. E.; Eisenberg, R.Inorg. Chem. 1989,28, 2423.
Teo, B.-K.; Snyder-Robinson, P. A.J. Chem. Soc., Chem. Commun. 1979, 255.
Calado, J. C. G.; Dias, A. R.; Martinho Simões, J. A.Rev. Port. Quim. 1979,21, 129.
Based on δf H m o(I, g)=106.76±0.04 kJ mol−1 and δf H m o(I2, g)=62.42±0.08 kJ mol−1 InCODATA Key Values for Thermodynamics, Cox, J. D.; Wagman, D. D.; Medvedev, A., Eds.; Hemisphere: New York, 1989.
Burke, N. E.; Singhal, A.; Hintz, M. J.; Ley, J. A.; Hui, H.; Smith, L. R.; Blake, D. M.J. Am. Chem. Soc. 1979,101, 74.
Drago, R. S.; Nozari, M. S.; Klinger, R. J.; Chamberlain, C. S.Inorg. Chem. 1979,18, 1254.
Martinho Simões, J. A.; Beauchamp, J. L.Chem. Rev. 1990,90, 629.
Minas da Piedade, M. E.; Martinho Simões, J. A.J. Organomet. Chem. 1996,518, 167.
Calhorda, M. J.; Veiros, L. F.J. Organomet. Chem. 1994,478 37, and references cited therein.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ciriano, M.A., Dias, A.R., Nunes, P.M. et al. Energetics of the oxidative addition of I2 to [Ir(Μ-L)(CO)2]2 (L=StBu, 3,5-Me2pz,7-aza) complexes. X-ray structures of Ir(Μ-StBu)(I)(CO)2]2 and [Ir(Μ-3,5-Me2pz)(I)(CO)2]2 . Struct Chem 7, 337–354 (1996). https://doi.org/10.1007/BF02275160
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02275160