Summary
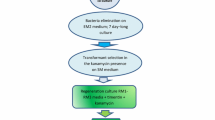
Agrobacterium-mediated transformation of thin cell layer explants (Klimaszewska and Keller 1985) yielded large numbers of transgenic plants of a major Canadian rapeseed cultivar Brassica napus ssp. oleifera cv Westar. The morphology and fertility of these plants were indistinguishable from controls. The Ti plasmid vector, pGV3850 (Zambryski et al. 1983) was used as a cis vector and as a helper plasmid for the binary vector pBin19 (Bevan 1984). Selectable marker genes that conferred resistance to high levels of kanamycin (Km) on Nicotiana tabacum were less efficient in the selection of transgenic B. napus. At low levels of Km (15 μg/ml) large numbers of transgenic plants (50%) were identified among the regenerants by nopaline synthase activity and several of these were confirmed by Southern blot analyses. Only a small number were resistant to higher levels of Km (80 μg/ml). Preliminary analyses indicated that resistance to Km was transmitted to the selfed progeny. Chimeric chloramphenicol acetyl transferase genes were ineffective biochemical markers in transgenic B. napus.
Similar content being viewed by others
References
Balazs E, Bonneville JM (1987) Chloramphenicol acetyl transferase activity in Brassica spp. Plant Sci 50:65–68
Bernatzky R, Tanksley SD (1986) Methods for detection of single or low copy sequences in tomato on Southern Blots. Plant Mol Biol Rep 4:37–91
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
De Block M, Herrera-Estrella L, Van Montagu M, Schell J, Zambryski P (1984) Expression of foreign genes in regenerated plants and in their progeny. EMBO J 3:1681–1689
Dunwell JM (1981) In vitro regeneration from excised leaf discs of three Brassica species. J Exp Bot 32:784–789
Guerche P, Jouanin L, Tepfer D, Pelletier G (1987) Genetic transformation of oilseed rape (Brassica napus) by the Ri T-DNA of Agrobacterium rhizogenes and analysis of inheritance of the transformed phenotype. Mol Gen Genet 206:382–386
Hain R, Stabel P, Czernilofsky AP, Steinbiss HH, Herrera-Estrella L, Schell J (1985) Uptake, integration, expression and genetic transmission of a selectable chimeric gene by plant protoplasts. Mol Gen Genet 199:161–168
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of VIR- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Holbrook LA, Miki BL (1985) Brassica crown gall tumouri-genesis and in vitro culture of transformed tissue. Plant Cell Rep 4:329–332
Holbrook LA, Haffner M, Miki BL (1986) A sensitive fluorographic method for the detection of nopaline and octopine synthase activities in Brassica crown-gall tissues. Biochem Cell Biol 64:126–132
Holsters M, Silva B, VanVliet F, Genetello C, De Block M, Dhaese P, Depicker A, Inzé D, Engler G, Villaroel R, Van Montagu M, Schell J (1980) The functional organization of the nopaline, Agrobacterium tumefaciens pTiC58. Plasmid 3:212–230
Horsch RB, Jones GE (1980) A double filter paper technique for plating cultured plant cells. In Vitro 16:103–108
Horsch RB, Fry JE, Hoffmann NL, Eicholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Kartha KK, Gamborg OL, Constabel F (1974) In vitro plant formation from stem explants of rape (Brassica napus cv Zephyr). Physiol Plant 31:217–220
Klimaszewska K, Keller WA (1985) High frequency plant regeneration from thin cell layer explants of Brassica napus. Plant Cell Tiss and Organ Cult 4:183–197
Lazzeri PA, Dunwell JM (1984a) In vitro shoot regeneration from seedling root segments of Brassica oleracea and Brassica napus cultivars. Ann Bot 54:341–350
Lazzeri PA, Dunwell JM (1984b) Establishment of isolated root cultures of Brassica species and regeneration from cultured root segments of Brassica oleracea var. italica. Ann Bot 54:351–361
Lichtenstein C, Draper J (1985) Genetic engineering of plants in DM Glover; DNA cloning, vol II. IRL Press, pp 67–120
Margara J, Leydecker M (1978) Différents types d'organogénèse observés chez le colza, Brassica napus L var. oleifera Metzg. Applications à la multiplication végétative in vitro. CR Acad Sci Paris 287:17–20
Mathews H, Bhatia CR, Mitra R, Krishna TG, Rao PS (1985) Regeneration of shoots from Brassica juncea (Linn) czern and coss cells transformed by Agrobacterium tumefaciens and expression of nopaline dehydrogenase genes. Plant Sci 39:49–54
Mathews H, Rao PS, Bhatia CR (1986) Transformation of Brassica juncea by Agrobacterium tumefaciens harbouring plasmid pTiT37 and its rooty mutant pTiT37.14a/a. J Genet 65:37–44
Ooms G, Bains A, Burrell M, Karp A, Twell D, Wilcox E (1985) Genetic manipulation in cultivars of oilseed rape (Brassica napus) using Agrobacterium. Theor Appl Genet 71:325–329
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rouselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Petit A, Tempe J (1978) Isolation of Agrobacterium Ti-plasmid regulatory mutants. Mol Gen Genet 166:147–155
Stringam GR (1979) Regeneration in leaf callus cultures of haploid rapeseed (Brassica napus L). Z Pflanzenphysiol 92:459–462
Tanaka N, Hayakawa M, Mano Y, Ohkawa H, Matsui C (1985) Infection of turnip and radish storage roots with Agrobacterium rhizogenes. Plant Cell Rep 4:74–77
Yarrow SA, Wu SC, Barsby TL, Kemble RJ, Shepard JF (1986) The introduction of CMS mitochondria to triazine tolerant Brassica napus L., var. Regent, by micromanipulation of individual heterokaryons. Plant Cell Rep 5:415–418
Zambryski P, Joos H, Genetello C, Leemans J, Van Montagu M, Schell J (1983) Ti-plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J 2:2143–2150
Author information
Authors and Affiliations
Additional information
Communicated by P. Maliga
Contribution No. 1092 Plant Research Centre, Ontario, Canada
Rights and permissions
About this article
Cite this article
Charest, P.J., Holbrook, L.A., Gabard, J. et al. Agrobacterium-mediated transformation of thin cell layer explants from Brassica napus L.. Theoret. Appl. Genetics 75, 438–445 (1988). https://doi.org/10.1007/BF00276747
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00276747