Abstract
Structural studies have been performed on lipid A preparations derived from lipopolysaccharides of nodulating (nod+) Rhizobium trifolii wild type strains and non-nodulating (nod-) mutants deprived of the medium size “Nod” plasmid.
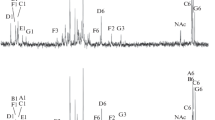
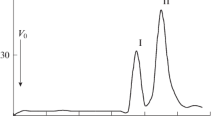
All preparations contain a lipid A backbone composed of a β1,6-linked d-glucosamine disaccharide (GlcN II-β1,6-GlcN I) which is bis-phosphorylated at positions 1 and 4′. The phosphate group at position 4′ (GlcN II) is nonstoichiometrically substituted probably by a neutral glycosyl residue. Another substituent (probably also a neutral sugar) substitutes partly position 4 (GlcN I) of the disaccharide. The hydroxyl groups at positions 3 and 3′ are likely to be esterified by fatty acyl residue. In lipid A of nod+ strains, 3-hydroxyhexa- and octadecanoic acid are linked to the amino group of GlcN I, and 3-hydroxyhexa- and tetradecanoic acid to the amino group of GlcN II. In lipid A of nod- strains, mainly 3-hydroxyhexadecanoic acid is linked to the amino group of GlcN I, and 3-hydroxytetra- and hexadecanoic acid to that of GlcN II.
The results show that rhizobial lipid A expresses many similarities to the lipid A of Enterobacteria. The structure shows, however, also peculiarities, especially regarding substituents of the lipid A backbone.
Similar content being viewed by others
References
Ball AK, Shantharan S (1981) Localization of primary and secondary lectin recetor sites in Rhizobium japonicum. In: Gibson DH, Newton WE (eds) Current perspectives in nitrogen fixation. Australian Academy of Science, Canberra, pp 422–422
Bock K, Pedersen C (1972) Reaction of sugar esters with hydrogen fluoride. Derivatives of d-glucofuranose and d-mannofuranose. Acta Chem Scand Ser B 26:2360–2366
Caroff M, Szabo L (1983) Identification of 2-amino-6-O-(2-amino-2-deoxy-β-d-glucopyranosyl)-2-deoxy-d-glucose as a major constitient of the hydrophobic region of the Bordetella pertussis endotoxin. Carbohydr Res 114:95–102
Dazzo FB, Brill WJ (1979) Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol 137:1362–1373
Dazzo FB, Hubbell DH (1975) Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol 30:1017–1033
Hakomori S (1964) Rapid permethylation of glycolipids and polysaccharides catalyzed by methyl sulfinyl carbonion in dimethyl sulfoxide. J Biochem Tokyo 55:205–208
Hase S, Rietschel E Th (1976) Isolation and analysis of the lipid A backbone. Lipid A structure of lipopolysaccharides from various bacterial groups. Eur J Biochem 63:101–107
Hrabak EM, Urbano MR, Dazzo FB (1981) Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind Trifoliin A, a white clover lectin. J Bacteriol 148:697–711
Jensen M, Borowiak D, Paulsen H, Rietschel E (1979) Analysis of permethylated glucosaminyl-glucosaminitol disaccharides by combined gas-liquid chromatography/mass spectrometry. Biomed Mass Spectrom 6:559–565
Kijne JD, Adhin SW, Planqué K (1977) Cell proliferation in pea root explants supplied with rhizobial LPS. In: Cell wall biochemistry related to specificity in host-plant pathogen interactions, Solheim B, Raa J (eds) Universitats-forlaget, Oslo, pp 415–416
Kowalczuk E, Skorupska A, Lorkiewicz Z (1981) Transfer of nodulation ability in Rhizobium using R68.45 derived plasmids. Mol Gen Genet 183:388–391
Lowry OH, Roberts NR, Leiner KY, Wu ML, Farr AL (1954) The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem 207:1–17
Maier RJ, Brill WJ (1978) Involvement of Rhizobium japonicum O antigen in soybean nodulation. J Bacteriol 133:1295–1299
Maier RJ, Bishop PE, Brill WJ (1978) Transfer from Rhizobium japonicum to Azotobacter vinelandii of genes required for nodulation. J Bacteriol 134:1199–1201
Mort AJ, Lamport DTA (1977) Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem 82:289–309
Napoli C, Dazzo F, Hubell D (1975) Production of cellulose microfibrils by Rhizobium. Appl Microbiol 30:123–131
Ohno K, Nishiyama H, Nagase H (1979) A mild methylation of alcohols with diazomethane catalyzed by silica gel. Tetrahed Lett 45:4405–4406
Planqué K, Kijne JW (1977) Binding of pea lectins to a glycan type polysaccharide in the cell walls of Rhizobium leguminosarum. FEBS Lett 73:64–66
Planqué K, Nierop JJ van, Burgers F, Wilkinson StG (1979) The lipopolysaccharide of free-living and bacteroid forms of Rhizobium leguminosarum. J Gen Microbiology 110:151–159
Qureshi N, Takayama K, Ribi E (1982) Purification and structural determination of nontoxic lipid A obtained from lipopolysaccharide of Salmonella typhimurium. J Biol Chem 257:11808–11815
Rietschel E Th, Hase S, King M, Redmond J, Lehman V (1977) The chemical structure of lipid A. In: Schlesinger D (ed) Microbiology 1977. Am Soc Microbiol, Washington DC, pp 262–268
Rietschel E Th, Wollenweber H-W, Russa R, Brade H, Zähringer U (1984) Concepts of the chemical structure of lipid A. Rev Inf Dis 6:432–438
Russa R, Lorkiewicz Z (1974) Fatty acids present in the lipopolysaccharide of Rhizobium trifolii. J Bacteriol 119:771–775
Russa R, Urbanik T, Kowalczuk E, Lorkiewicz Z (1982) Correlation between the occurrence of plasmid pUCS202 and lipopolysaccharide alterations in Rhizobium. FEMS Microbiol Letters 13:161–1654
Sidorczyk Z, Zähringer U, Rietschel E Th (1983) Chemical structure of the lipid A component of the lipopolysaccharide from a Proteus mirabilis Re mutant. Eur J Biochem 137:15–22
Strominger JL, Park JT, Thompson RE (1959) Composition of the cell wall of Staphylococcus aureus: Its relation to the mechanism of action of penicillin. J Biol Chem 234:3263–3268
Sweeley CC, Bentley R, Makita M, Wells WW (1963) Gas-liquid chromatography of trimethylsilyl derivatives of sugar and related substances. J Am Chem Soc 85:2497–2507
Tharanathan RN, Weckesser J, Mayer H (1978) Structural studies on the d-arabinose-containing lipid A from Rhodospirillum tenue 2761. Eur J Biochem 84:395–394
Westphal O, Jann K (1965) Bacterial lipopolysaccharides, extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem 5:83–91
Wollenweber HW, Broady KW, Lüderitz O, Rietschel E Th (1982) The chemical structure of lipid A: demonstration of amidelinked 3-acyloxyacyl-residues in Salmonella minnesota Relipopolysaccharide. Eur J Biochem 124:191–198
Wolpert JS, Albersheim P (1976) Symbiont interactions. I. The lectins of legumes interact with the O antigen-containing lipopolysaccharide of their symbiont Rhizobia. Biochem Biophys Res Commun 70:729–737
Zajac E, Russa R, Lorkiewicz Z (1975) Lipopolysaccharide as receptor for Rhizobium phage IP. J Gen Microbiol 90:365–367
Zeevenhuizen LPTM, Sholten-Koerselman I, Postumus MA (1980) Lipopolysaccharides of Rhizobium. Arch Microbiol 125:1–8
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Professor Dr. Gerhart Drews on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Russa, R., Lüderitz, O. & Rietschel, E.T. Structural analyses of lipid A from lipopolysaccharides of nodulating and non-nodulating Rhizobium trifolii . Arch. Microbiol. 141, 284–289 (1985). https://doi.org/10.1007/BF00428838
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00428838