Abstract
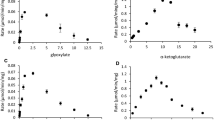
Serine transhydroxymethylase of Methanobacterium thermoautotrophicum has been purified to within 95% of homogeneity. Activity was strictly dependent on tetrahydromethanopterin, tetrahydrofolate being unable to serve as the acceptor C1 units from l-serine. The native protein has a molecular weight of about 102,000 daltons. The enzyme shows maximal activity at 60°C, has a pH optimum of 8.1, and required pyridoxal-5′-phosphate and Mg2+ for optimal activity.
Similar content being viewed by others
References
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol 59:20–31
Eirich LD, Vogels GD, Wolfe RS (1978) Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry 17: 4583–4593
Escalante-Semerena JC, Leigh JA, Wolfe RS (1984a) New insights into the biochemistry of methanogenesis from H2 and CO2. In: Crawford RL, Hanson RS (eds) Proceedings of the 4th symposium on Microbial Growth on C1 Compounds. Am Soc Microbiol Publications, Washington, DC, pp 191–198
Escalante-Semerena JC, Leigh JA, Reinhart KL Jr, Wolfe RS (1984b) Formaldehyde activation factor, tetrahydromethanopterin, a coenzyme involved in methanogensis. Proc Natl Acad Sci USA 81:1976–1980
Escalante-Semerena JC, Reinhart KL Jr, Wolfe RS (1984c) Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem 259:9447–9455
Escalante-Semerena JC, Wolfe RS (1984) Formaldehyde oxidation and methanogenesis. J Bacteriol 158:721–726
Fairbanks J, Steck TL, Wallach DFH (1971) Electophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606–2617
Gunsalus RP, Wolfe RS (1980) Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. J Biol Chem 255:1891–1895
Gunsalus RP, Tandom SM, Wolfe RS (1980) A procedure for anaerobic column chromatography employing an anaerobic, Fretertype chamber. Anal Biochem 101:327–331
Hedrick JL, Smith AJ (1968) Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Anal Biochem 126:155–164
Jungermann KA, Schmidt W, Kirchnawy FH, Rupprecht EH, Thauer RK (1970) Glycine formation via threonine and serine aldolase. Its interaction with the pyruvate formate lyase pathway of one-carbon unit synthesis in Clostridium kluyveri. Eur J Biochem 16:424–429
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Leigh JA (1983) Levels of water-soluble vitamins in methanogenic and non-methanogenic bacteria. Appl Environ Microbiol 45:800–803
Leigh JA, Wolfe RS (1983) Carbon dioxide reduction factor and methanopterin, two coenzymes required for CO2 reduction to methane by extracts of Methanobacterium. J Biol Chem 258:7536–7540
Mansouri A, Decter JB, Silber R (1972) Studies on the regulation of one-carbon metabolism. II. Repression-derepression of serine transhydroxymethylase in Escherichia coli 113-3. J Biol Chem 247:348–352
McBride BC, Wolfe RS (1971) Biochemistry of methane formation. In: Anaerobic biological treatment processes. Advances in Chemistry Series no. 105. American Chemical Society, pp 11–22
Merrill CR, Goldman D, Sedman SA, Ebert MA (1981) Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science 211:1437–1438
O'Connor ML, Hanson RS (1975) Serine transhydroxymethylase isozymes from a facultative methylotroph. J Bacteriol 124: 985–966
Romesser JA, Wolfe RS (1982) Coupling of methyl coenzyme M reduction with carbon dioxide activation in extracts of Methanobacterium thermoautotrophicum. J Bacteriol 152:840–847
Schirch L (1971) Serine transhydroxymethylase (rabbit liver). In: Tabor H, Tabor CW (eds) Methods in enzymology, vol 27B. Academic Press, New York, pp 335–340.
Schirch LG, Mason M (1963) Serine transhydroxymethylase. A study of the properties of a homogeneous enzyme preparation and of the nature of its interaction with substrates and pyridoxal-5′-phosphate. J Biol Chem 238:1032–1037
Schirch V, Hopkins S, Villar E, Angelaccio S (1985) Serine transhydroxymethylase from Escherichia coli: purification and properties. J Bacteriol 163:1–7
Sutherland E, Cori C, Haynes R, Olsen N (1949) Purification of the hyperglycemic-glycogenolytic factor from insulin and gastric mucosa. J Biol Chem 180:825–837
Taylor RT, Weissbach H (1965) Radioactive assay for serine transhydroxymethylase. Anal Biochem 13:80–84
Uyeda K, Rabinowitz JC (1968) Enzymes of the clostridial purine fermentation. Serine transhydroxymethylase. Arch Biochem Biophys 123:271–278
Van Beelen P, Stassen APM, Bosch JWG, Vogels GD, Guijt W, Haasnoot CAG (1984) Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear magnetic resonance techniques. Eur J Biochem 138:563–571
Whitman WB, Ankwanda E, Wolfe RS (1982) Nutrition and carbon metabolism in Methanococcus voltae. J Bacteriol 149:852–863
Wood JM, Allam AM, Brill WJ, Wolfe RS (1965) Formation of methane from serine by cell-free extracts of Methanobacillus omelianskii. J Biol Chem 240:4554–4569
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoyt, J.C., Oren, A., Escalante-Semerena, J.C. et al. Tetrahydromethanopterin-dependent serine transhydroxymethylase from Methanobacterium thermoautotrophicum . Arch. Microbiol. 145, 153–158 (1986). https://doi.org/10.1007/BF00446773
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00446773