Abstract
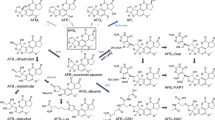
In vitro studies with rat liver parenchymal, Kupffer and endothelial cells isolated from male Sprague-Dawley rats were undertaken to investigate cell-specific bioactivation of aflatoxin B1, DNA binding and adduct formation. In the mutagenicity studies, using homogenates of all three separated liver cell populations (co-incubated with NADP+ and glucose-6-phosphate as cofactors for the cytochrome P-450 monooxygenase system) parenchymal, Kupffer and endothelial cells were able to activate aflatoxin B1 to a metabolite mutagenic to Salmonella typhimurium TA 98. In the case of nonparenchymal cells (i.e. Kupffer and endothelial cells) 10-fold higher concentrations of aflatoxin B1 had to be used to obtain a similar number of revertants to that observed with parenchymal cells. Induction studies with Aroclor 1254 led to a striking decrease in the activation of aflatoxin B1 in parenchymal cells, whereas nonparenchymal cells had a slightly enhanced metabolic activation capacity for aflatoxin B1. Metabolism studies with microsomes from induced and noninduced cells using testosterone as substrate revealed comparable results: after induction with Aroclor 1254, parenchymal cells showed a 60% decrease in the formation rate of 2α-hydroxytestosterone, whereas the formation rate of this metabolite remained unchanged in nonparenchymal cells; 2α-hydroxytestosterone is specifically formed by cytochrome P-450 IIC11, which also catalyses the activation of aflatoxin B1 to its epoxide. When freshly isolated, intact cells were incubated with tritiated aflatoxin B1, a dose-dependent aflatoxin B1 binding to DNA in parenchymal and nonparenchymal cells was observed. HPLC analysis of DNA acid hydrolysates of all three cell types showed the major adduct to be 8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1.
Similar content being viewed by others
Abbreviations
- AFB1 :
-
aflatoxin B1
- AFB1-gua:
-
8,9-dihydro-8-(N7-guanyl)-9-hydroxy-aflatoxin B1
References
Appleton S, Goetchius MP, Campell TC (1982) Linear dose-response curve for the hepatic macromolecular binding of aflatoxin B1 in rats at very low exposures. Cancer Res 42: 3659–3662
Autrup H, Essigmann JM, Croy RG, Trump BF, Wogan GN, Harris CC (1979) Metabolism of aflatoxin B1 and identification of the major aflatoxin B1-DNA adducts formed in cultured human bronchus and colon. Cancer Res 39: 694–698
Baertschi SW, Raney KD, Stone MP, Harris TM (1988) Preparation of the 8,9-epoxide of the mycotoxin aflatoxin B1: the ultimate carcinogenic species. J Am Chem Soc 110: 7929–7931
Battista JR, Marnett LJ (1985) Prostaglandin H synthase-dependent epoxidation of aflatoxin B1. Carcinogenesis 6: 1227–1229
Brouwer A, Barelds RJ, Knook DL (1984) Centrifugal separation of mammalian cells. In: Rickwood D (ed) Centrifugation, a practical approach. IRL Press, Oxford, p 183
Coles B, Meyer DJ, Ketterer B, Stanton CA, Garner RC (1985) Studies on the detoxication of microsomally activated aflatoxin B1 by glutathione and glutathione transferases in vitro. Carcinogenesis 6: 693–697
Croy RG, Wogan GN (1981) Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res 41: 197–203
Degen GH, Neumann H (1981) Differences in aflatoxin B1-susceptibility of rat and mouse are correlated with the capability in vitro to inactivate aflatoxin B1-epoxide. Carcinogenesis 2: 299–306
Essigmann JM, Croy RG, Busby WF Jr, Reinhold VN, Büchi G, Wogan GN (1977) Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci USA 74: 1870–1874
Garner RC, Martin CN, Lindsay Smith JR, Coles BF, Toison MR (1979) Comparison of aflatoxin B1, and aflatoxin G1 binding to cellular macromolecules in vitro, in vivo and after peracid oxidation, Characterisation of the major nucleic acid adducts. Chem Biol Interact 26: 57–73
Glatt HR, Billings R, Platt KL, Oesch F (1981) Improvement of the correlation of bacterial mutagenicity with carcinogenicity of benzo-(a) pyrene and four of its major metabolites by activation with intact liver cells instead of homogenate. Cancer Res 41: 270–277
Gurtoo HL, Dahms RP, Paigen B (1978) Metabolic activation of aflatoxins related to their mutagenicity. Biochem Biophys Res Commun 81: 965–972
Hertzog PJ, Smith JRL, Garner RC (1980) A high pressure liquid chromatography study on the removal of DNA-bound aflatoxin B1 in rat liver and in vitro. Carcinogenesis 1: 787–793
Holeski CJ, Eaton DL, Monroe DH, Bellamy GM (1987) Effects of phenobarbital on the biliary excretion of aflatoxin P1-glucuronide and aflatoxin B1-S-glutathione in the rat. Xenobiotica 17: 139–153
Ishii K, Maeda K, Kamataki T, Kato R (1986) Mutagenic activation of aflatoxin B1 by several forms of purified cytochrome P-450. Mutat Res 174: 85–88
Jorgensen KV, Clayton JW, Price RL (1987) Evaluation of aflatoxin B1 mutagenesis: addition of glutathione and glutathione-S-transferase to the Salmonella mutagenicity assay. Environ Mutagen 9: 411–419
Kalengayi MMR, Desmet VJ (1975) Sequential histological and histochemical study of the rat liver during aflatoxin B1-induced carcinogenesis. Cancer Res 35: 2845–2852
Kitada M, Taneda M, Die H, Komori M, Itahashi K, Nagao M, Kamataki J (1989) Mutagenic activation of aflatoxin B1 by P-450 HFLa in human fetal livers. Mutat Res 227: 53–58
Koser PL, Faletto MB, Maccubbin AE, Gurtoo HL (1988) The genetics of aflatoxin B1 metabolism. J Biol Chem 263: 12584–12595
Lafranconi WM, Glatt HR, Oesch F (1986) Xenobiotic metabolizing enzymes of rat liver nonparenchymal cells. Toxicol Appl Pharmacol 84: 500–511
Levin W, Thomas PE, Reick LM, Wood AW, Ryan DE (1984) Multiplicity and functional diversity of rat hepatic microsomal cytochrome P-450 isozymes. In: Paton W, Mitchell J, Turner P (eds) Proceedings of IUPHAR 9th International Congress of Pharmacology Vol. 3. McMillan, London, p 203
Lewis JG, Swenberg JA (1980) Differential repair of O6-methylguanine in DNA of rat hepatocytes and nonparenchymal cells. Nature 288: 185–187
Metcalfe S, Neal GE (1983) Some studies on the relationship between the cytotoxicity of aflatoxin B1 to rat hepatocytes and metabolism of the toxin. Carcinogenesis 4: 1013–1019
Newberne PM, Wogan GN (1968) Sequential morphologic changes in aflatoxin B1 carcinogenesis in the rat. Cancer Res 28: 770–781
Newberne PM, Butler WH (1969) Acute and chronic effects of aflatoxin B1 on the liver of domestic and laboratory animals: a review. Cancer Res 29: 236–250
Shimada T, Guengerich FP (1989) Evidence for cytochrome P-450 NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci USA 86: 462–465
Shimada T, Nakamura S, Imaoka S, Funae Y (1987) Genotoxic and mutagenic activations of aflatoxin B1 by constitutive forms of cytochrome P-450 in rat liver microsomes. Toxicol Appl Pharmacol 91: 13–21
Steinberg P, Lafranconi WM, Wolf CR, Waxman DJ, Oesch F, Friedberg T (1987) Xenobiotic metabolizing enzymes are not restricted to parenchymal cells in rat liver. Mol Pharmacol 32: 463–470
Van der Hoeven T (1981) Testosterone oxidation by rat liver microsomes: effects of phenobarbital pretreatment and the detection of seven metabolites by HPLC. Biochem Biophys Res Commun 100: 1285–1291
Weibel ER, Stäubli W, Gnägi RH, Hess FA (1969) Correlated morphometric and biochemical studies on the liver cell. 1. Morphometric model, stereological methods and normal morphometric data for rat liver. J Cell Biol 42: 68–91
Wild CP, Garner RC, Montesano R, Tursi F (1986) Aflatoxin B1 binding to plasma albumin and liver DNA upon chronic administration to rats. Carcinogenesis 7: 853–858
Wogan GN (1973) Aflatoxin carcinogenesis. In: Busch H (ed) Methods in cancer research. Academic Press, New York, p 309
Wogan GN, Newberne PM (1967) Dose-dependent characteristics of aflatoxin B1 carcinogenesis in the rat. Cancer Res 27: 2370–2376
Yeowell HN, Waxman DJ, Wadhera A, Goldstein JA (1987) Suppression of the constitutive, male-specific rat hepatic cytochrome P-450 2c and its mRNA by 3,4,5,3′,4′,5′-hexachlorobiphenyl and 3-methyl-cholanthrene. Mol Pharmacol 32: 340–347
Author information
Authors and Affiliations
Additional information
This project was supported by the Deutsche Forschungsgemeinschaft (SFB 302). B. Schlemper was the recipient of a European Science Foundation Scholarship
Rights and permissions
About this article
Cite this article
Schlemper, B., Harrison, J., Garner, R.C. et al. DNA binding, adduct characterisation and metabolic activation of aflatoxin B1 catalysed by isolated rat liver parenchymal, Kupffer and endothelial cells. Arch Toxicol 65, 633–639 (1991). https://doi.org/10.1007/BF02098028
Issue Date:
DOI: https://doi.org/10.1007/BF02098028