Abstract
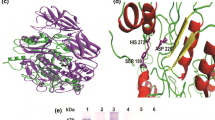
A cDNA encoding a novel human putative member of the papain family of cysteine peptidases has been cloned. The protease, named cathepsin P, is synthesized as a preproprotein. The presumed propeptide of 38 amino acids is followed by a 242-residue mature protein. The mature protease region is 30% identical to human papain-like cathepsins, with all the residues important for catalysis conserved. No similarity was observed in the propeptide region. On the contrary, the proenzyme shares 51-87% residues with some precursors of cysteine proteases from other species that have not yet been characterized. They all show a nearly completely conserved “CYTRED motif” in the propeptide region, not present in other members of the family, and could therefore constitute a distinct subfamily.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Published: January 2000
Rights and permissions
About this article
Cite this article
Pungerčar, J., Ivanovski, G. Identification and molecular cloning of cathepsin P, a novel human putative cysteine protease of the papain family. Pflügers Arch 439 (Suppl 1), r116–r118 (2000). https://doi.org/10.1007/s004240000112
Issue Date:
DOI: https://doi.org/10.1007/s004240000112