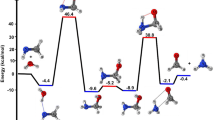
Abstract
Theoretical ab initio calculations are done on different mechanisms for the conversion of vinyl alcohol to acetaldehyde, both in gas phase and in solution. Several basis sets are used in order to assess the accuracy of the results in gas phase and a continuum model of the solvent is employed to mimic reactions in water solution. The results indicate a catalytic action of water in hydrated clusters in gas phase, whereas in solution, and within the error limits of our calculations, both neutral water-chain and ionic mechanisms appear to be equally probable. Finally, the action of acids or bases is tested through the analysis of the reaction of vinyl alcohol with H3O+ and HO−. The results of the calculations are shown to be in qualitative agreement with the experimental facts when 6-31++G basis set is used but not when either STO-3G or 4-31G basis sets are employed.
Similar content being viewed by others
References and notes
Lledós A, Bertrán J, Ventura ON Int J Quantum Chem, in press
Lledós A, Bertrán J (1981) Tetrahedron Lett 775
Bertrán J, Lledós A, Revellat JA (1983) Int J Quantum Chem 23:587
Lledós A, Bertrán J (1984) J Mol Struct 107:233
Lledós A, Bertrán J (1985) J Mol Struct 120:73
Ventura ON, Bartolucci JP (1984) Theor Chim Acta 64:229 and references therein
Muñiz MA, Bertrán J, Andrés JL, Durán M, Lledós A (1985) J Chem Soc Faraday Trans 1 81:1547
Newton MD (1977) J Chem Phys 67:5535
Toullec J (1982) Adv Phys Org Chem 18:1
Hart H (1979) Chem Rev 79:515
Saito S (1976) Chem Phys Lett 42:399
Blank B, Fischer H (1973) Helv Chim Acta 56:506
Blank B, Henne A, Laroff GP, Fischer H (1975) Pure Appl Chem 41:475
Capon B, Rycroft DS, Watson TW, Zucco C (1981) J Am Chem Soc 103:1761
Capon B, Zucco C (1982) J Am Chem Soc 104:7567
Guthrie JP, Cullimore PA (1979) Can J Chem
Guthrie JP (1979) Can J Chem 57:797
Guthrie JP (1979) Can J Chem 57:1177
Hehre WJ, Stewart RF, Pople JA (1969) J Chem Phys 51:2657
Ditchfield R, Hehre WJ, Pople JA (1971) J Chem Phys 54:724
Radom L (1977) Mod Theor Chem 4:333
Chandrasekhar J, Andrade JG, Ragué-Schleyer PV (1981) J Am Chem Soc 103:5609 and references therein
Cao HZ, Tapia O, Evleth EM (1985) J Phys Chem 89:1581
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
Hehre WJ, Ditchfield R, Pople JA (1972) 56:2257
Boggs JE, Cordell FR (1981) J Mol Struct 76:329
Flament JP (1984) J Chim Phys 81:673
Likas SG, Liebman JF, Levin RD (1984) J Phys Chem Ref Data 13:695
Schlegel HB (1982) J Comp Chem 3:214
Schlegel HB (1984) Theor Chim Acta 66:333
Binkley JS, Whiteside RA, Krishnan R, Seeger R, Defrees DI, Shlegel HB, Topiol S, Kahn LR,Pople JA (1980) QCPE 13:406
Fletcher R (1980) Practical methods of optimization, vol. 1. Wiley, Chichester
Peterson MR, Poirier RA: MONSTERGAUSS, Department of Chemistry, University of Toronto, Canada
Miertuš S, Scrocco E, Tomasi J (1981) Chem Phys 55:117
Hehre WJ, Lathan WA, Ditchfield R, Newton MD, Pople JA (1973) QCPE 11:236
Bonaccorsi R, Palla P, Tomasi J (1984) J Am Chem Soc 106:1945
In a typical calculation, no more than 10 points were skipped due to this safeguard
Pierotti RA (1976) Chem Rev 76:717
Halicioglu T, Sinanoglu O (1969) Ann NY Acad Sci 158:308
Hirschfelder JO, Stevenson PP, Eyring H (1937) J Chem Phys 5:896
Bondi A (1954) J Phys Chem 58:929
Dugben AJ, Miertus S (1982) Chem Phys Lett 88:395
Huron MJ, Claverie P (1972) J Phys Chem 76:2123
Miertus S, Tomasi J (1982) Chem Phys 65:239
Bonaccorsi R, Cimiraglia R, Tomasi J (1983) Chem Phys Lett 99:77
Moelwyn-Hughes EA 1965 Physical chemistry. Pergamon Press, Oxford
Buttery RG, Ling LC, Guadangni DG (1969) J Agro Food Chem 17:385
Cabani S, Gianni P, Mollica V, Lepori L (1981) J Solution Chem 10:563
Spangler D, Williams IH, Maggiora GM (1983) J Comput Chem 4:524
Clavero C, Lledós A, Durán M, Ventura ON, Bertrán J (1986) J Am Chem Soc 108:923
Clavero C, Lledós A, Durán M, Ventura ON, Bertrán J J Comput Chem: to be published
Rodwell WR, Bouma WJ, Radom L (1980) Int J Quantum Chem 18:107
Interatomic Distances Supplement, Esp. Publ. No 18, Chem Soc, London, 1965
Harmony MD, Laurie VW, Kuezkowski RL, Schwendeman RH, Ramsay DA, Lovas FJ, Lafferty WJ, Maki AG (1979) J Phys Chem Ref Data 8:619
Symons MCR (1980) J Am Chem Soc 102:3982
Blank B, Fischer H (1973) Helv Chim Acta 56:506
Kilb RW, Lin CC, Wilson Jr EB (1957) J Chem Phys 26:1695
Pascual Ahuir JL, Silla E, Tomasi J, Bonaccorsi R: to be published
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ventura, O.N., Lledós, A., Bonaccorsi, R. et al. Theoretical study of reaction mechanisms for the ketonization of vinyl alcohol in gas phase and aqueous solution. Theoret. Chim. Acta 72, 175–195 (1987). https://doi.org/10.1007/BF00527661
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00527661