Abstract
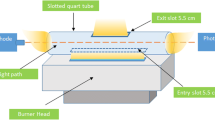
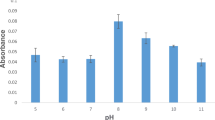
A 23 factorial experimental design has been used to determine the optimum reaction conditions for organotin ethylation in simple aqueous solutions for speciation by Cryogenic Trapping/ Gas Chromatography/Atomic Absorption Spectrometry (CT/GC/AAS). The factors chosen and their levels of variation (− and +) were the pH of the solution (levels 3 and 5), the amount of NaBEt4 added (levels 1 and 9 mg) to the organotins and the time of ethylation reaction (levels 10 and 20 min). Optimum conditions were investigated for monomethyltin (MMT), diethyltin (DET) and dibutyltin (DBT) in mixed solution at a concentration of 10 ng of each Sn compound in 50 ml of solution (200 ng·l−1). The influence of the different factors on the yield of the ethylation reaction in the ranges investigated depends on the degree of substitution and the nature of the alkyl groups of the organotins. The ethylation reaction for DET and DBT is more efficient at high pH levels, MMT gives higher yields at lower pH. Both MMT and DET require a high amount of reagent, while reagent concentration has no real influence on the DBT signal. Comparison of hydride generation and ethylation as derivatisation procedures for organotin speciation has been performed in simple solutions. Under these analytical conditions, hydride generation is shown to be slightly more sensitive than ethylation by a factor of 1.4 for MMT and DET and 2 for DBT. However derivatisation using ethylation provides more reproducible results and is not affected by inorganic interferents.
Similar content being viewed by others
References
Craig PJ (ed) (1986) Organometallic compounds in the environment. Longman Group, Harlow, 368 pp
Rapsomanikis S, Weber JH (1986) In: Craig PJ (ed) Organometallic compounds in the environment, Longman Group, Harlow, pp 279–303
Donard OFX, Martin FM (1992) Trends Anal Chem 11:17
Donard OFX (1989) In: Sonderdruck aus Colloquium Atomspektrometrische Spuren Analytik (5), Bodenseewerk, Perkin-Elmer, Uberlingen p 395
Matthias CL, Olson GJ, Brinckman FE, Bellama JM (1986) Environ Sci Technol 20:609
Valkirs AO, Seligman PF, Stang PM, Homer V, Lieberman SH, Vafa G, Dooley CA (1986) Mar Pollut Bull 17:319
Dirkx WMR, Van Mol WE, Van Cleuvenbergen RJA, Adams FC (1989) Fresenius Z Anal Chem 335:769
Mueller MD (1987) Anal Chem 59:617
Maguire RJ, Tkacz RJ (1983) J Chromatogr 268:99
Dezausiers V, Leguille F, Lavigne R, Astruc M, Pinel R (1989) Appl Organomet Chem 3:431
Quevauviller Ph, Martin FM, Belin C, Donard OFX (1993) Appl Organomet Chem 7:149
Martin FM: Tseng C-M, Belin C, Quevauviller P, Donard OFX (1993) Anal Chim Acta 286:343
Martin FM, Donard OFX (1994) J Anal Atom Spectrom (in press)
Rapsomanikis S, Donard OFX, Weber JH (1986) Anal Chem 58:35
Bloom N (1989) Can J Fish Aquat Sci 40:1131
Rapsomanikis S and Craig PJ (1991) Anal Chim Acta 248:563
D'ulivo A, Chen Y (1989) J Anal Atom Spectrom 4:319
Ashby J, Craig PJ (1991) Appl Organomet Chem 5:173
Ashby J, Craig PJ (1989) Sci Total Environ 78:219
Ashby J, Clark S, Craig PJ (1988) J Anal Atom Spectrom 3:735
Michel P, Averty B (1991) Appl Organomet Chem 5:393
Cai Y, Rapsomanikis S, Andreae MO (1993) Anal Chim Acta 274:243
Cai Y, Rapsomanikis S, Andreae MO (1993) J Anal Atom Spectrom 8:119
Clark S, Craig PJ (1992) Mikrochim Acta 109:141
Cochran WG, Cox GM (1957) Experimental design, 2nd ed. Wiley, New York, pp 611
Box GEP, Hunter WG, Hunter JS (1978) Statistics for experiments. Wiley, New York
Goupy J (1988) La méthode des plans d'expériences, Dunod (ed), 303 pp
Honeycutt JB Jr, Riddle JM (1961) J Am Chem Soc 83:369
Cotton FA, Wilkinson G (1988) Chapter nine: The group IVA (14) elements: Si, Ge, Sn, Pb. In: Advanced inorganic chemistry, 5th ed. Wiley, New York, pp. 265–304
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, F.M., Donard, O.F.X. Aqueous ethylation of organotin compounds in simple solution for speciation analysis by cryofocussing and detection by atomic absorption spectrometry — comparison with hydride generation. Fresenius J Anal Chem 351, 230–236 (1995). https://doi.org/10.1007/BF00321643
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00321643