Summary
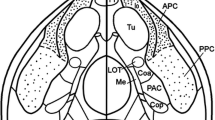
Electrophysiological methods were employed to study the axonal properties of the neurons of anterior olfactory nucleus (AON), transition zone (TZ), and rostral prepyriform cortex (RPPC) and their projections towards the ipsilateral and contralateral olfactory bulb (IOB, COB) in the rat.
Of 91 antidromically driven cells, 39 (43%) and 32 (35%) responded to IOB and COB stimulation, respectively; 20 (22%) were discharged from both bulbs. Collision tests performed on the latter group indicated that these neurons have a short main axon which divides near the soma, projecting one branch to the COB and a thinner one toward the IOB. Mean conduction velocities of axons projecting to the IOB and the COB were 0.4 m/s and 0.7 m/s, respectively, the faster conducting axons having shorter refractory periods.
Of the 38 neurons tested, 92% showed decreases in threshold and latency (up to 20% of control antidromic latency) after a test volley that was preceded by a conditioning pulse at intervals of 20–215 ms. Latency decreases were greater for slowly conducting axons than for the faster ones. These after-effects of impulse activity in OB afferent axons were attributed to the presence of a supernormal period of increased conduction velocity and excitability similar to that found in the olfactory nerve (Bliss and Rosenberg, 1974).
Similar content being viewed by others
References
Bagshaw E V, Evans M H (1976) Measurement of current spread from microelectrodes when stimulating within the nervous system. Exp Brain Res 25: 391–400
Biedenbach M A, Stevens C F (1969) Electrical activity in cat olfactory cortex produced by synchronous orthodromic volleys. J Neurophysiol 32: 193–203
Bliss T V P, Rosenberg M E (1974) Supranormal conduction velocity in the olfactory nerve of the tortoise. J Physiol (Lond) 239: 60–61
Broadwell R D, Jacobowitz D M (1976) Olfactory relationships of the telencephalon and diencephalon in the rabbit. III. The ipsilateral centrifugal fibres to the olfactory bulbar and retrobulbar formations. J Comp Neurol 170: 321–345
Daval G, Levêteau J (1974) Electrophysiological studies of centrifugal and centripetal connections of the anterior olfactory nucleus. Brain Res 78: 395–410
Davis, B J, Macrides F, Youngs W H, Schneider S P, Rosene D L (1978) Efferents and centrifugal afferents of the main and accessory olfactory bulb in the hamster. Brain Res Bull 3: 59–72
Dennis B J, Kerr D L B (1976) Origins of olfactory bulb centrifugal fibres in the cat. Brain Res 110: 593–600
de Olmos J S, Hardy H, Heimer L (1978) The afferent connections of the main and the accessory olfactory bulb formations in the rat. J Comp Neurol 181: 213–244
Faiers A A, Mogenson G J (1976) Electrophysiological identification of neurons in locus coeruleus. Exp Neurol 53: 254–266
Freeman W J (1968) Relations between unit activity and evoked potentials in prepyriform cortex of cats. J Neurophysiol 31: 337–348
Fuller J H, Schlag J D (1976) Determination of antidromic excitation by the collision test: Problems of interpretation. Brain Res 112: 283–298
Haberly L B (1973) Unitary analysis of opossum prepyriform cortex. J Neurophysiol 36: 762–774
Haberly L B, Price J L (1978) Association and commissural fibre systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol 181: 781–807
Heimer L, de Olmos J (1978) Club program, Cajal Meeting, April 2, 1978 Vancouver, Canada
Hursh J B (1939) Conduction velocity and diameter of nerve fibers. Am J Physiol 127: 131–139
Mori K, Satou M, Takagi S F (1979) Axonal projection of anterior olfactory nuclear neurons to the olfactory bulb bilaterally. Exp Neurol 64: 295–305
Raymond S A, Lettvin J Y (1978) Aftereffects of activity in peripheral axons as a clue to nervous coding. In: Waxman S G (ed) Physiology and pathobiology of axons. Raven Press, New York, pp 203–225
Scalia F, Winans S S (1975) The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161: 31–56
Schafa F, Meisami E (1977) A horseradish peroxidase study of the origin of central projections to the rat olfactory bulb. Brain Res 136: 355–359
Scholfield C N (1978) Electrical properties of neurons in the olfactory cortex slice in vitro. J Physiol (Lond) 275: 535–546
Shinoda Y, Ghez C, Arnold A (1977) Spinal branching of rubrospinal axons in the cat. Exp Brain Res 30: 203–218
Swadlow H A (1974) Systematic variations in the conduction velocity of slowly conducting axons in the rabbit corpus callosum. Exp Neurol 43: 445–451
Swadlow H A, Waxman S G (1976) Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol 53: 128–150
Swadlow H A, Waxman S G (1978) Activity-dependent variations in the conduction properties of central axons. In: Waxman S G (ed) Physiology and pathobiology of axons. Ravan Press, New York, pp 191–202
Swadlow H A, Waxman S G, Rosene D L (1978) Latency variability and the identification of antidromically activated neurons in mammalian brain. Exp Brain Res 32: 439–443
Tolbert D L, Bantli H, Bloedel J R (1976) Anatomical and physiological evidence for a cerebellar nucleo-cortical projection in the cat. Neuroscience 1: 205–217
Tolbert D L, Bantli H, Bloedel J R (1978) Multiple branching of cerebellar efferent projections in cats. Exp Brain Res 31: 305–316
Valverde F (1965) Studies on the piriform lobe. Harvard University Press, Cambridge, MA
Walsh R R (1959) Olfactory bulb potentials evoked by electrical stimulation of the contralateral bulb. Am J Physiol 196: 327–329
Waxman S G, Swadlow H A (1976) Morphology and physiology of visual callosal axons: evidence for a supernormal period in central myelinated axons. Brain Res 113: 179–187
Westrum L E (1970) Observations on initial segments of axons in the prepyriform cortex of the rat. J Comp Neurol 139: 337–356
Willey T J, Maeda G, Rafuse D (1975) Antidromic units in the prepyriform cortex driven by olfactory peduncular volleys. Brain Res 92: 132–136
Author information
Authors and Affiliations
Additional information
This work was supported by a grant, “Convenio de Cooperatión Científico-Tecnológica, Argentino-Norteamericano, No. 7363/ 75”, provided by the Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina
Rights and permissions
About this article
Cite this article
Ferreyra Moyano, H., Molina, J.C. Axonal projections and conduction properties of olfactory peduncle neurons in the rat. Exp Brain Res 39, 241–248 (1980). https://doi.org/10.1007/BF00237113
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00237113