Summary
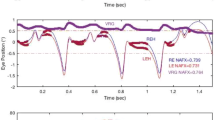
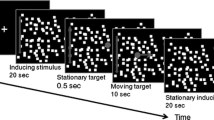
It was previously proposed that a linkage between the optokinetic system and the stereoscopic system in higher mammals serves to allow these animals to selectively stabilize those parts of the visual scene which lie in the plane of convergence as the animals move forward in a three dimensional world (Howard and Ohmi, 1984). A new procedure is now described by which OKN gain can be measured as a function of the binocular disparity of the stimulus. With vergence locked on a vertical line, the gain of the slow phase of vertical optokinetic nystagmus (OKN) was recorded in four human subjects as the binocular disparity (stereo depth) of the moving display was changed from-3° to + 3°. The gain of OKN was found to be inversely proportional to binocular disparity. Evidence for cells in the visual cortex, MT and MST that are sensitive both to visual motion and binocular disparity is reviewed. It is argued that the activity of cells responsive to direction of motion and zero disparity selectively augments OKN and that this enables humans to stabilize the images of parts of the scene in the plane of regard while ignoring competing motion signals arising from other distances.
Similar content being viewed by others
References
Anstis SM, Harris JP (1974) Movement aftereffects contingent on binocular disparity. Perception 3:153–168
Büttner U, Waespe W (1984) Purkinje cell activity in the primate flocculus during optokinetic stimulation, smooth pursuit eye movements and VOR-suppression. Exp Brain Res 55:97–104
Collewijn H (1976) Impairment of optokinetic (after) nystagmus by labyrinthectomy in the rabbit. Exp Neurol 52:146–156
Collewijn H (1975) Direction-selective units in the rabbit's nucleus of the optic tract. Brain Res 100:489–508
Fox R, Patterson R, Lehmkule S (1982) Effect of depth position on the motion aftereffect. Invest Ophthal Vis Sci (ARVO Suppl) 22:144
Hine T (1985) The binocular contribution to monocular optokinetic nystagmus and after nystagmus asymmetries in humans. Vision Res 25:589–598
Hoffmann KP (1982) Cortical versus subcortical contributions to the optokinetic reflex in the cat. In: Lennerstrand G, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon Press, New York, pp 303–311
Hoffmann KP, Distler C (1986) The role of direction selective cells of the nucleus of the optic tract of cat and monkey during optokinetic nystagmus. In: Keller EL, Zee DS (eds) Adaptive processes in vision and oculomotor systems. Pergamon, New York, pp 261–267
Howard IP (1961) An investigation of a satiation process in the reversible perspective of revolving skeletal shapes. Q J Exp Psychol 13:19–31
Howard IP, Gonzalez EG (1987) Human optokinetic nystagmus in response to moving binocularly disparate stimuli. Vision Res 27:1807–1816
Howard IP, Ohmi M (1984) The efficiency of the central and peripheral retina in driving human optokinetic nystagmus. Vision Res 24:969–976
Komatsu H, Roy JP, Wurtz RH (1988) Binocular disparity sensitivity of cells in area MST of the monkey. Soc Neurosci Abstr 14:202
Mackensen G (1953) Untersuchungen zur Physiologie des optokinetischen Nystagmus. Klin Mdl Augenheilk 123:133–143
Maunsell JHR, Van Essen DC (1983) Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol 49:1148–1167
Mehdorn E (1982) Nasal-temporal asymmetry of the optokinetic nystagmus after bilateral occipital infarction in man. In: Lennerstrand G, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon, Oxford, pp 321–324
Montarolo W, Precht W, Strata P (1981) Functional organization of the mechanisms subserving the optokinetic nystagmus in the cat. Neuroscience 6:231–246
Petersik JT, Shepard A, Malsch R (1984) A three-dimensional motion aftereffect produced by prolonged adaptation to a rotation simulation. Perception 13:489–497
Poggio GF, Fischer B (1977) Binocular interaction and depth sensitivity of striate and prestriate cortical neurons of the behaving rhesus monkey. J Neurophysiol 40:1392–1405
Poggio GF, Talbot WH (1981) Mechanisms of static and dynamic stereopsis in foveal cortex of the rhesus monkey. J Physiol (London) 315:469–492
Regan D, Cynader M (1982) Neurons in cat visual cortex tuned to the direction of motion in depth: effect of stimulus speed. Invest Ophthal Vis Sci 22:535–550
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Bio-Med Engin BME-10:137–145
Schiff B, Cohen T, Raphan T (1988) Nystagmus induced by stimulation of the nucleus of the optic tract in the monkey. Exp Brain Res 70:1–14
Schor C and Levi DM (1980) Disturbances of small-field horizontal and vertical optokinetic nystagmus in amblyopia. Invest Ophthal Vis Sci 19:668–683
Smith RA (1976) The motion/disparity aftereffect: a preliminary study. Vision Res 16:1507–1509
Suzuki DA, Keller EL (1984) Visual signals in the dorsolateral pontine nucleus of the alert monkey: their relationship to smooth-pursuit eye movements. Exp Brain Res 53:473–478
Toyama K, Komatsu H, Kasai H, Fujii K, Umetani K (1985) Responsiveness of Clare-Bishop neurons to visual cues associated with motion of a visual stimulus in three-dimensional space. Vision Res 25:407–414
Tychen L, Lisberger (1986) Maldevelopment of visual motion processing in humans who had strabismus with onset in infancy. J Neurosci 6:2495–2508
Tyler CW, Scott AB (1979) Binocular vision. In: Records RE (ed) Physiology of the human eye and visual system. Harper and Row, New York, pp 643–674
van Hof-Van Duin J, Mohn G (1982) Stereopsis and optokinetic nystagmus. In: Lennerstrand G, Zee DS, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon, Oxford, pp 113–119
Waespe W, Henn V (1987) Gaze stabilization in the primate: the interaction of the vestibulo-ocular reflex, optokinetic nystagmus, and smooth pursuit. Rev Physiol Biochem Pharmacol 106:38–125
Waespe W, Rudinger D, Wolfensberger M (1985) Purkinje cell activity in the flocculus of vestibular neurectomized and normal monkeys during optokinetic nystagmus (OKN) and smooth pursuit eye movements. Exp Brain Res 60:243–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Howard, I.P., Simpson, W.A. Human optokinetic nystagmus is linked to the stereoscopic system. Exp Brain Res 78, 309–314 (1989). https://doi.org/10.1007/BF00228902
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228902