Summary
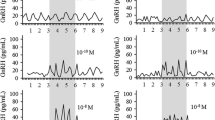
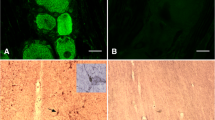
Gamma-aminobutyric acid (GABA) is thought to play an important role in the regulation of luteinizing hormone-releasing hormone (LHRH) release but its role in the regulation of LHRH gene expression and LHRH synthesis is not known. We hypothesized that since GABA appears to have primarily inhibitory effects on LHRH cells (at the level of the cell body), GABA may act to decrease LHRH gene expression and peptide synthesis. This hypothesis was tested by examining the effect of GABA receptor activation and GABA receptor blockade on LHRH mRNA and peptide levels employing in situ hybridization and immunocytochemistry. Cells in the preoptic area (POA) of ovariectomized (ovx) rats were selectively exposed in vivo to specific GABA-ergic receptor agonists or an antagonist for up to 24 h. THIP, a specific GABA a receptor agonist, did not have a significant effect on either the intensity of LHRH immunoreactivity, or the number of LHRH-ir cells, observed as compared to controls. Baclofen, a GABA b receptor agonist appeared to decrease the number of cells with the greatest intensity of LHRH immunoreactivity, compared to controls. In situ hybridization, with either a tritiated RNA probe or a 32P-labelled oligonucleotide, complementary to LHRH mRNA, revealed that THIP either had no effect on the labelling intensity (32P-labelled oligonucleotide) or (contrary to our hypothesis) a slight excitatory effect on the level of LHRH mRNA detected per cell (tritiated RNA probe). Bicuculline (a specific GABA a receptor antagonist) decreased both the number of labelled cells observed per section through the POA, and the intensity of labelling observed in sections hybridized with the 32P-labelled oligonucleotide. These results suggest that in the POA GABA a receptors do not exert an inhibitory effect on LHRH gene expresssion, but rather could affect LH perhaps by electrically inhibiting LHRH neurons. In contrast, baclofen appeared to exert an inhibitory effect on LHRH gene expression, since the number of grains per labelled cell in the POA of baclofen treated rats was lower than the grains per labelled cell of control rats. Also, similar to the results obtained with immunocytochemistry, in situ hybridization following baclofen treatment suggested that activation of GABA b receptors is able to reduce the number of neurons with the highest levels of LHRH mRNA. Thus, in the POA, GABA acting through GABA b receptors could be effective through changes in mRNA or peptide synthesis.
Similar content being viewed by others
References
Adler BA, Crowley WR (1986) Evidence for gamma-aminobutyric acid modulation of ovarian hormonal effects on luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinology 118:91–97
Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M (1980) (-) Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature 283:92–94
Bowery NG, Hudson AL, Price GW (1987) GABA a and GABA b receptor site distribution in the rat central nervous system. Neuroscience 20:365–383
Demling J, Fuchs E, Baumert M, Wuttke W (1985) Preoptic catecholamine, GABA, and glutamate release in ovariectomized and ovariectomized estrogen-primed rats utilizing push-pull cannula technique. Neuroendocrinology 41:212–218
Donoso AO (1988) Localized increase of GABA levels in brain areas of the rat and inhibition of the plasma LH rise following ovariectomy. Neuroendocrinology 48:130–137
Donoso AO, Munoz V, Banzan AM (1986) Inhibitory actions of GABA on luteinizing hormone secretion. In: Racagni G, Donoso AO (eds) GABA and endocrine function. Raven Press, New York, pp 191–199
Enna SJ, Karbon EW (1986) GABA Receptors: An overview. In: Olsen RW, Ventor JC (eds) Benzodiazepine/GABA receptors and chloride channels: structural and functional properties. Alan Liss, New York, pp 41–56
Fahrbach SE, Morrell J, Pfaff DW (1984) Temporal pattern of HRP spread from an iotophoretic site and description of a new HRP-Gel implant method. J Comp Neurol 225:605–619
Flügge G, Oertel WH, Wuttke W (1986) Evidence for estrogen-receptive GABA-ergic neurons in the preoptic/anterior hypothalamic area of the rat. Neuroendocrinology 43:1–5
Fuchs E, Mansky T, Stock K-W, Vijayan E, Wuttke W (1984) Involvement of catecholamines and glutamate in GABAergic mechanism regulatory to luteinizing hormone and prolactin secretion. Neuroendocrinology 38:484–489
Hartman RD, He J-R, Barraclough CA (1990) γ-aminobutyric acid-A and -B receptor antagonists increase luteinizing hormone-releasing hormone neuronal responsiveness to intracerebroventricular norepinephrine in ovariectomized estrogentreated rats. Endocrinology 127:1336–1345
Hejtmancik HJ, Conn PM, Pfaff DW (1989) In situ hybridization for detecting gonadotropin-releasing hormone messenger RNA and measuring physiologically stimulated changes. In: Conn PM (ed) Methods in neuroscience, Vol 1. Gene probes. Academic Press, New York, pp 208–222
Herbison AE, Heavens RP, Dyer RG (1989) Oestrogen and nor-adrenaline modulate endogenous GABA release from slices of the rat medial preoptic area. Brain Res 486:195–200
Herbison AE, Heavens RP, Dyer RG (1990) Oestrogen modulation of excitatory Al noradrenergic input to rat medial preoptic GABA neurones demonstrated by microdialysis. Neuroendocrinology 52:161–168
Hollander M, Wolfe DA (1973) Nonparametric statistical methods. John Wiley and Sons, New York
Jarry H, Perschl A, Wuttke W (1988) Further evidence that preoptic anterior GABA-ergic neurons are part of the GnRH pulse and surge generator. Acta Endocrinol (Copenh) 118:573–579
Johnston GAR (1986) Multiplicity of GABA receptors. In: Olsen RW, Ventor JC (eds) Benzodiazepine/GABA receptors and chloride cannels: structural and functional properties. Alan Liss, New York, pp 57–71
Lamberts R, Vijayan E, Graf M, Mansky T, Wuttke W (1983) Involvement of preoptic-anterior hypothalamic GABA neurons in the regulation of pituitary LH and prolactin release. Exp Brain Res 52:356–362
Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F (1985) Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 40:536–539
Mansky T, Mestres-Ventura P, Wuttke W (1982) Involvement of GABA in the feedback action of estradiol on gonadotropin and prolactin release: hypothalamic GABA and catecholamine turnover rates. Brain Res 231:353–364
Masotto C, Wisniewski G, Negro-Vilar A (1989) Different gammaaminobutyric acid receptor subtypes are involved in the regulation of opiate-dependent and independent luteinizing hormone-releasing hormone secretion. Endocrinology 125:548–553
Masotto C, Negro-Vilar A (1986) GABA and gonadotropin secretion: evidence from in vitro studies on regulation of LHRH secretion. In: Racagni G, Donoso AO (eds) GABA and endocrine function. Raven Press, New York, pp 243–250
Masotto C, Negro-Vilar A (1987) Activation of gammaaminobutyric acid B-receptors abolishes naloxone-stimulated luteinizing hormone release. Endocrinology 121:2251–2255
Negro-Vilar A, Vijayan E, McCann SM (1980) The effect of intraventricular gamma-aminobutyric acid (GABA) on hypothalamic catecholamines and LHRH and on pituitary hormone release. Brain Res Bull 5:239–244
Nikolarakis KE, Loeffler J-Ph, Almeida OFX, Herz A (1988) Preand post-synaptic actions of GABA on the release of hypothalamic gonadotropin-releasing hormone (GnRH). Brain Res Bull 21:677–683
Ondo J, Mansky T, Wuttke W (1982) In vivo GABA release from the medial preoptic area of diestrous and ovariectomized rats. Exp Brain Res 46:69–72
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates 2nd edn. Academic Press, Sydney
Rothfeld J, Hejtmancik JF, Conn PM, Pfaff DW (1989) In situ hybridization for LHRH mRNA following estrogen treatment. Molec Brain Res 6:121–125
Schwanzel-Fukuda M, Garcia MS, Morrell JI, Pfaff DW (1987) Distribution of luteinizing hormone-releasing hormone in the nervous terminalis and brain of the mouse detected by immunocytochemistry. J Comp Neurol 255:231–244
Schwanzel-Fukuda M, Morrell JI, Pfaff DW (1983) Polyacrylamide gel provides slow release delivery of wheat germ agglutinin (WGA) for retrograde neuroanatomical tracing. J Histochem Cytochem 31:831–836
Seeburg PH, Adelman JP (1984) Characterization of cDNA for precursor of human luteinizing hormone releasing hormone. Nature 311:666–668
Sokal RR, Rohlf FJ (1981) Biometry 2nd edn. WH Freeman, San Fransisco
Uhl G (1986) In situ hybridization in brain. Plenum Press, New York
Vijayan E, McCann SM (1978) Re-evaluation of the role of catecholamines in control of gonadotropin and prolactin release. Neuroendocrinology 25:150–165
Vincent SR, Hokfelt T, Wu J-Y (1982) GABA neuron systems in hypothalamus and the pituitary gland. Neuroendocrinology 34:117–125
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergen, H.T., Hejtmancik, J.F. & Pfaff, D.W. Effects of gamma-aminobutyric acid receptor agonists and antagonist on LHRH-synthesizing neurons as detected by immunocytochemistry and in situ hybridization. Exp Brain Res 87, 46–56 (1991). https://doi.org/10.1007/BF00228505
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228505