Summary
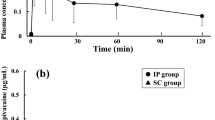
The pharmacokinetic constants and rectal bioavailability of the narcotic analgesic ketobemidone were determined in six male patients after surgery. Plasma concentrations were measured following intravenous administration of Ketogin® 2 ml, containing ketobemidone chloride 10 mg, and a spasmolytic compound N,N-dimethyl-3,3-diphenyl-1-methylallylamine chloride 50 mg, and following rectal administration of one suppository of Ketogin®, containing ketobemidone chloride 10 mg and the spasmolytic component 50 mg. Following intravenous administration, the disposition of ketobemidone followed a biexponential pattern with a fast distribution phase and a slower elimination phase: the plasma half-life (t1/2β) was 2.42±0.41 h (rodel ± SD). After rectal administration, the disposition of ketobemidone fitted a one-compartment model. The elimination half-life was 3.27±0.32 h. The mean rectal bioavailability for ketobemidone was 44%±9%. The pharmacokinetic constants of the spasmolytic component, N,N-dimethyl-3,3-diphenyl-1-methylallylamine, were also determined in five of the patients, both after intravenous and after rectal administration. The plasma half-life was 3.07±0.53 h and 3.79±1.14 h, respectively. The rectal bioavailability was estimated to be 33%±14%.
Similar content being viewed by others
References
Bondesson U, Arnér S, Anderson P, Boréus LO, Hartvig P (1980) Clinical pharmacokinetics and oral bioavailability of ketobemidone. Eur J Clin Pharmacol 17: 45–50
Bondesson U, Hartvig P (1979) Mass fragmentographic method for the determination of ketobemidone in plasma. J Chromatogr 179: 207–212
de Boer AG, Breimer DD, Mattie M, Pronk J, Gubbens-Stibbe JM (1979) Rectal bioavailability of lidocaine in man: Partial avoidance of “first-pass” metabolism. Clin Pharmacol Ther 26: 701–709
Tamsen A, Bondesson U, Dahlström B, Hartvig P (1980) Patient controlled analgesic therapy (PACAT) with ketobemidone. Clin Pharmacokinet (accepted for publication)
Petersen PV (1951) Studies on a new spasmolytic compound 1,1-diphenyl-3-dimethylaminobutene-1 (A29), related to methadone, and on the combined use of this compound and a potent analgesic, ketobemidone (A21). Acta Pharmacol Toxicol 7: 51–64
Boréus LO (1971) Use of human fetal ileum for evaluation of smooth muscle effects of narcotic analgesics. Eur J Pharmacol 15: 127–131
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, P., Arnér, S., Bondesson, U. et al. Clinical pharmacokinetics of ketobemidone. Its bioavailability after rectal administration. Eur J Clin Pharmacol 19, 217–223 (1981). https://doi.org/10.1007/BF00561953
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00561953