Summary
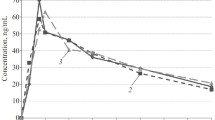
The pharmacokinetics of subcutaneous dihydroergotamine (DHE) with or without dextran 70 infusion was evaluated in a single- and multiple-dose study in 30 patients. Radioimmunoassay was used to measure plasma DHE and the anthrone method to determine the dextran concentration. In the single-dose study no significant interaction between DHE and dextran was noted with respect to their plasma levels. The absorption of s.c. DHE was rapid and the disappearance curve followed a biphasic pattern, t0.5 α being 1.4 and 2.0 h, t0.5 β 22 and 21 h for DHE and DHE/dextran 70, respectively. In the multi-dose study the trough level of DHE initially had a tendency to rise, in accordance with simulated plasma concentration curves. DHE trough levels were about 0.5 ng/ml and were well above the assumed minimum effective value to induce venoconstriction (0.06 ng/ml). Dextran concentrations were significantly higher when DHE was co-administered, possibly, due to changes in plasma volume. It is concluded that DHE 0.5 mg s.c. twice daily will give an adequate plasma concentration and that there was no important interaction between it and infused dextran 70.
Similar content being viewed by others
References
Åberg M, Arfors K-E, Bergentz S-E (1977) Effect of dextran on factor VIII and thrombus stability in humans. Significance of varying infusion rates. Acta Chir Scand 143: 417–419
Aellig WH, Nüesch E (1977) Comparative pharmacokinetics investigations with tritium-labelled ergot alkaloids after oral and intravenous administration in man. Int J Clin Pharmacol 15: 106–112
Bergqvist D, Efsing HO, Hallböök T, Lindblad B (1980) Prevention of postoperative thromboembolic complications. A prospective comparison between dextran 70, dihydroergotamine heparin and a sulphated polysaccharid. Acta Chir Scand 146: 559–568
Bobik A, Skews H, Jennings G, Esler M, Korner PI (1980) Dihydroergotamine kinetics in patients with orthostatic hypotension. Clin Exp Pharmacol Physiol 7: 684
Buttermann G, Theisinger W, Oechsler H, Hör G (1975) Untersuchungen über die postoperative Thromboembolie-Prophylaxe nach einem neuen medikamentösen Behandlungsprinzip. Dtsch Med Wochenschr 100: 2065–2069
Gauer OH (1976) Moderator's introduction. Cardiology 61: [Suppl 1] 2–6
Hilke H, Kanto J, Mäntylä R, Kleimala T, Syvälahti E (1978) Dihydroergotamine: Pharmacokinetics and usefulness in spinal anaesthesia. Acta Anaesthesiol Scand 22: 215–220
Jenner H (1967) Automated determination of the molecular weight distribution of dextran. 3rd European Technicon Symposium Brighton, England, p 203–207
Kunz S, Drähne A, Briel RC (1977) Prophylaxe der postoperativen Thromboembolie-Erfahrungen mit Heparin Dihydergot® in der Gynäkologie. In: Pabst HW, Maurer G (ed) Postoperative Thromboembolie-Prophylaxe. 6. Rothenburger Gespräch 20–21 Mai 1977. Schattauer, Stuttgart, pp 134–144
Lamke LO, Liljedahl SO (1976) Plasma volume changes after infusion of various plasma expanders. Resuscitation 5: 93–106
Lange LM, Echt M (1972) Vergleichende Untersuchungen über venentonisierende Pharmaka. Fortschr Med 90: 1161–1173
Little PJ, Jennings GL, Skews H, Bobik A (1982) Bioavailability of dihydroergotamine in man. Br J Clin Pharmacol 13: 785–790
Meier J, Schreier E (1976) Human plasma levels of some antimigraine drugs. Headache 16: 96–104
Mühe E, Burghardt K-H, Kolb W, Strobel G (1975) Eine neue Methode zur Prophylaxe postoperativer Venenthrombosen. Klinikarzt 4: 88–92
Müller-Schweinitzer E (1980) In vitro studies on the duration of action of dihydroergotamine. Int J Clin Pharmacol Ther Toxicol 18: 88–91
Olver I, Jennings G, Bobik A, Esler M (1980) Low bioavailability as a cause of apparent failure of dihydroergotamine in orthostatic hypotension. Br Med J 2: 275–276
Rosenthaler J, Munzer H (1976) 9,10-Dihydroergotamine: Production of antibodies and radioimmunoassay. Experientia (Basel) 32: 234–236
Sagar S, Stamatakis JD, Higgins AF, Nairn D, Maffei FH, Thomas DP, Kakkar VV (1976) Efficacy of low-dose heparin in prevention of extensive deep-vein thrombosis in patients undergoing total hip replacement. Lancet 2: 1151–1154
Sheiner LB (1981) ELSFIT. A program for the extended least squares FIT to individual pharmacokinetic data. Users manual. A technical report of the Division of Clinical Pharmacology of California, SF, CA 94143
Stamatakis JD, Sagar S, Lawrence D, Kakkar VV (1977) Dihydroergotamine in the prevention of postoperative deep venous thrombosis. Br J Surg 64: 294
Thorén L (1978) Dextran as a plasma volume substitute. Progr Clin Biol Res 19: 265–282
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindblad, B., Abisch, E. & Bergqvist, D. The pharmacokinetics of subcutaneous dihydroergotamine with and without a dextran 70 infusion. Eur J Clin Pharmacol 24, 813–818 (1983). https://doi.org/10.1007/BF00607093
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00607093