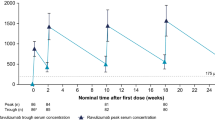
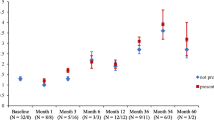
Summary
The pharmacokinetics and development of antinuclear antibodies (ANAs) during procainamide (PA) therapy were studied in 35 patients with ventricular arrhythmias. Sixteen of the subjects were rapid and 19 were slow acetylators. Twenty-six of them (13 rapid and 13 slow acetylators) received PA therapy (2.4 g sustained-release PA·HCl daily in three doses) for at least 16 weeks. On maintenance therapy, rapid acetylators had insignificantly lower serum PA concentrations and slightly higher N-acetylprocainamide (NAPA) concentrations than slow acetylators. The unchanged PA fraction (PA/PA + NAPA) in the rapid acetylators was somewhat lower than in the slow acetylators. Rapid acetylators excreted more NAPA in urine than did slow acetylators (p<0.05), whereas the difference in PA excretion was not significant. More than 80% of the given drug was excreted as PA and NAPA. Spontaneous or exercise-induced arrhythmias were recorded in 6 rapid and 8 slow acetylators. ANAs (titre at least 20) appeared in 6 rapid and 8 slow acetylators. The mean time until ANA development in rapid acetylators was only marginally longer than in slow acetylators. The results suggest that acetylation phenotyping is not of great significance in predicting the development of ANAs during PA therapy.
Similar content being viewed by others
References
Atkinson AJ Jr, Lee W-K, Quinn ML, Kushner W, Nevin MJ, Strong JM (1977) Dose-ranging trial of N-acetylprocainamide in patients with premature ventricular contractions. Clin Pharmacol Ther 21: 575–587
Atkinson AJ Jr, Strong JM (1977) Effect of active drug metabolites on plasma level-response correlations. J Pharmacokin et Biopharm 5: 95–109
Birkhead J, Evans T, Mumford P, Martinez E, Jewitt D (1976) Sustained release procainamide in patients with myocardial infarction. Br Heart J 38: 77–80
Bratton AC, Marshall EK Jr (1939) A new coupling component for sulfanilamide determination. J Biol Chem 128: 537–550
Campbell W, Tilston WJ, Lawson DH, Hutton I, Lawrie TDV (1976) Acetylator phenotype and the clinical pharmacology of slow-release procainamide. Br J Clin Pharmacol 3: 1023–1026
Davies DM, Beedie MA, Rawlins MD (1975) Antinuclear antibodies during procainamide treatment and drug acetylation. Br Med J 3: 682–683
Frislid K, Berg M, Hansteen V, Lunde PKM (1976) Comparison of the acetylation of procainamide and sulfadimidine in man. Eur J Clin Pharmacol 9: 433–438
Gibsona TP, Matusik J, Matusik E, Nelson HA, Wilkinson J, Briggs WA (1975) Acetylation of procainamide in man and its relation to isonicotinic acid hydrazide acetylation phenotype. Clin Pharmacol Ther 17: 395–399
Graffner C, Johnsson G, Sjögren J (1975) Pharmacokinetics of procainamide intravenously and orally as conventional and slow-release tablets. Clin Pharmacol Ther 17: 414–423
Henningsen NC, Cederberg A, Hanson A, Johannson BW (1975) Effects of long-term treatment with procaine amide. Acta Med Scand 198: 475–482
Karlsson E (1975) Procainamide and phenytoin. Comparative study of their antiarrhythmic effects at apparent therapeutic plasma levels. Br Heart J 37: 731–740
Karlsson E (1978) Clinical pharmacokinetics of procainamide. Clin Pharmacokinet 3: 97–107
Karlsson E, Molin L (1975) Polymorphic acetylation of procaine amide in healthy subjects. Acta Med Scand 197: 299–302
Karlsson E, Molin L, Norlander B, Sjöqvist F (1974) Acetylation of procaine amide in man studied with a new gas chromatographic method. Br J Clin Pharmacol 1: 467–475
Kluger J, Drayer D, Reidenberg M, Ellis G, Lloyd V, Tyber T, Hayes J (1980) The clinical pharmacology and antiarrhythmic efficacy of acetylprocainamide in patients with arrhythmias. Am J Cardiol 45: 1250–1257
Koch-Weser J (1977) Serum procainamide levels as therapeutic guides. Clin Pharmacokinet 2: 389–402
Koch-Weser J, Klein SW (1979) Procainamide dosage schedules, plasma concentrations and clinical effects. J Am Med Assoc 215: 1454–1460
Koch-Weser J, Klein SW, Foo-Canto LL, Kastor JA, Descantis RW (1969) Antiarrhythmic prophylaxis with procaine amide in acute myocardial infarction. N Engl J Med 281: 1253–1260
Lee W-K, Strong JM, Kehoe RF, Dutcher JS, Atkinson AJ Jr (1976) Antiarrhythmic efficacy of N-acetylprocainamide in patients with premature ventricular contractions. Clin Pharmacol Ther 19: 508–514
Lertora JJL, Atkinson AJ Jr, Kushner W, Nevin MJ, Lee W-K, Jones C, Schmid FR (1979) Long-term antiarrhythmic therapy with N-acetylprocainamide. Clin Pharmacol Ther 25: 273–282
Lunde PKM, Frislid K, Hansteen V (1977) Disease and acetylation polymorphism. Clin Pharmacokinet 2: 182–197
Matusik E, Gibson TP (1975) Fluorometric assay for N-acetylprocainamide. Clin Chem 21: 1899–1902
Reidenberg MM, Drayer DE, Levy M, Warner H (1975) Polymorphic acetylation of procainamide in man. Clin Pharmacol Ther 17: 722–730
Roden DM, Reele SB, Higgins SB, Wilkinson GR, Smith RF, Oates JA, Woosley RL (1980) Antiarrhythmic efficacy, pharmacokinetics and safety of N-acetylprocainamide in human subjects: Comparison with procainamide. Am J Cardiol 46: 463–468
Ruosteenoja R, Torsti P, Sothmann A (1973) Experiences on a sustained-release procainamide preparation. Curr Ther Res 15: 707–712
Schröder P, Klitgaard NA, Simonsen E (1979) Significance of the acetylation phenotype and the therapeutic effect of procainamide. Eur J Clin Pharmacol 15: 63–68
Sonnhag C, Karlsson E (1979) Comparative antiarrhythmic efficacy of intravenous N-acetylprocainamide and procainamide. Eur J Clin Pharmacol 15: 311–317
Sonnhag C, Karlsson E, Hed J (1979) Procainamide-induced lupus erythematosus-like syndrome in relation to acetylator phenotype and plasma levels of procainamide. Acta Med Scand 206: 245–251
Stec PG, Atkinson AJ Jr, Nevin MJ, Thenot J-P, Ruo TI, Gibson TP, Ivanovich P, del Greco F (1979) N-acetylprocainamide pharmacokinetics in functionally anephric patients before and after perturbation by hemodialysis. Clin Pharmacol Ther 26: 618–628
Strong JM, Dutcher JS, Lee W-K, Atkinson AJ Jr (1975a) Absolute bioavailability in man of N-acetylprocainamide determined by a novel stable isotope method. Clin Pharmacol Ther 18: 613–622
Strong JM, Dutcher JS, Lee W-K, Atkinson AJ Jr (1975b) Pharmacokinetics in man of the N-acetylated metabolite of procainamide. J Pharmacokinet Biopharm 3: 223–235
Tilstone WJ, Lawson DH, Campbell W, Hutton I, Lawrie TDV (1978) The pharmacokinetics of slow-release procainamide. Eur J Clin Pharmacol 14: 261–265
Wierzchowiecki M, Michalowska D, Lowicki Z, Ochotny R, Grześkowiak A, Tomaszkiewicz T (1980) Pharmacokinetic studies of procainamide (PA) and N-acetylprocainamide (NAPA) in healthy subjects. Int J Clin Pharmacol Ther Toxicol 18: 272–276
Wilson JD (1980) Antinuclear antibodies and cardiovascular drugs. Drugs 19: 292–305
Woosley RL, Drayer DE, Reidenberg MM, Nies AS, Carr K, Oates JA (1978) Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med 21: 1157–1159
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ylitalo, P., Ruosteenoja, R., Leskinen, O. et al. Significance of acetylator phenotype in pharmacokinetics and adverse effects of procainamide. Eur J Clin Pharmacol 25, 791–795 (1983). https://doi.org/10.1007/BF00542522
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00542522