Abstract
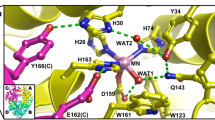
Human copper-cobalt superoxide dismutase in the reduced form has been investigated through 1H NMR techniques. The aim is to monitor the structural properties of this derivative and to compare them with those of reduced and oxidized native superoxide dismutases. The observed signals of the cobalt ligands have been assigned as well as the signals of the histidines bound to copper(I). The latter signals experience little pseudocontact shifts which allow a rough orientation of the magnetic susceptibility tensor in the molecular frame. The connectivities indicate that, although the histidine bridge is broken in the reduced form, the interproton distances between ligands of both ions are essentially the same.
Similar content being viewed by others
Abbreviations
- WEFT:
-
water eliminated Fourier transform
- NOE:
-
nuclear Overhauser effect
- NOESY:
-
NOE spectroscopy
- COSY:
-
correlation spectroscopy
- TOCSY:
-
total correlation spectroscopy
- SOD:
-
superoxide dismutase
- E2Co(II)SOD:
-
SOD with empty copper site (E=empty) and with cobalt(II) in the Zinc(II) site
References
Aue WP, Bartholdi E, Ernst RR (1976) Two dimensional spectroscopy. Application to nuclear magnetic resonance. J Chem Phys 64:2225–2246
Banci L, Bertini I, Luchinat C, Monnanni R, Scozzafava A (1988) Water 1H nuclear magnetic relaxation dispersion (NMRD) of Cu2Zn2SOD with some anions and 1H NMR spectra of Cu2Co2SOD in the presence of CN−. Inorg Chem 27:107–109
Banci L, Bertini I, Luchinat C, Piccioli M, Scozzafava A, Turano P (1989a) 1H NOE studies on copper(II)2 cobalt(II)2 superoxide dismutase Inorg Chem 28:4650–4656
Banci L, Bertini I, Luchinat C, Scozzafava A (1989 b) Cyanide and azide behave in a similar fashion versus cupro-zinc superoxide dismutase. J Biol Chem 264:9742–9744
Banci L, Bencini A, Bertini I, Luchinat C, Piccioli M (1990a) 1H NOE and ligand field studies of superoxide dismutase with anions. Inorg Chem 29:4867–4873
Banci L, Bencini A, Bertini I, Luchinat C, Viezzoli MS (1990 b) The angular overlap analysis of the spectroscopic parameters of copper-zinc SOD and its mutants. Gazz Chim Ital 120:179–185
Banci, L, Bertini I, Luchinat C, Viezzoli MS (1990c) A comment on the 1H NMR spectra of cobalt (II) substituted superoxide dismutase with histidines deuteriated in position ε1. Inorg Chem 29:1438–1440
Banci L, Bertini I, Luchinat C, Piccioli M (1991) Frontiers in NMR of Paramagnetic Molecules: 1H NOE and Related experiments: In: Bertini I, Molinari H, Niccolai, N (eds) NMR and biomolecular structure. VCH, Weinheim, pp 31–60
Beem KM, Richardson DC, Rajagopalan KV (1977) Metal sites of copper-zinc superoxide dismutase. Biochemistry 16:1930–1936
Bertini, I, Luchinat C (1986) NMR of paramagnetic molecules in biological systems. Benjamin and Cummings, Menlo Park, NY
Bertini, I, Lanini G, Luchinat C, Messori L, Monnanni R, Scozzafava A (1985 a) An investigation of Cu2Co2SOD and its anion derivatives. J Am Chem Soc 107:4391–4396
Bertini I, Luchinat C, Monnanni R (1985b) Evidence of the breaking of the copper-imidazolate bridge in copper cobalt-substituted superoxide dismutase upon reduction of the copper(II) centers. J Am Chem Soc 107:2178–2179
Bertini I, Banci L, Luchinat C, Hallewell RA (1988) The exploration of the active site cavity of copper-zinc superoxide dismutase. Ann NY Acad Sci 542:37–52
Bertini I, Banci L, Luchinat C, Hallewell RA (1988) The exploration of the active site cavity of copper-zinc superoxide dismutase. Ann NY Acad Sci 542:37–52
Bertini I, Banci L, Luchinat C, Piccioli M (1990) Spectroscopies studies Cu2Zn2SOD: a continuous advancement of investigation tools. Coord Chem Rev 100:67–103
Bertini I, Capozzi F, Luchinat C, Piccioli M, Viezzoli MS (1991) Assignment of active site protons in the 1H NMR spectrum of reduced human Cu, Zn superoxide dismutase. Eur J Biochem 197:691–697
Beyer WF, Fridovich I, Mullenbach GT, Hallewell RA (1987) Examination of the role of arginine-143 in the human copper and zinc superoxide dismutase by site-specific mutagensis. J Biol Chem 262:11182–11187
Blackburn NJ, Hasnais SS, Diakun GP, Knowles PF, Binsted N, Garner CD (1983) An extended X-ray absorption fine-structure study of the copper and the zinc sites of freeze-dried bovine superoxide dismutase. Biochem J 213:765–768
Blackburn NJ, Hasnais SS, Binsted N, Diakun GP, Garner CD, Knowles PF (1984) An extended X-ray absorption fine-structure study of bovine superoxide dismutase in aqueous solution. Biochem J 219:985–990
Brigg RG, Fee JA (1978) Further characterization of human erythrocyte superoxide dismutase. Biochem Biophys Acta 537:86–99
Cass AEG, Hill HAO, Smith BE, Bannister JV, Bannister WH (1977) Carbon-2 proton exchange at histidine-41 in bovine erythrocyte superoxide dismutase. Biochem J 165:587–589
Emerson SD, La Mar GN (1990) NMR determination of the magnetic susceptibility tensor in cianometmyoglobin: a new probe of steric tilt of bound ligand. Biochemistry 29:1556–1666
Fee JA (1973) Studies on the reconstitution of bovine erythrocyte superoxide dismutase. IV: Preparation and some properties of the enzyme in which Co (II) is substituted by Zn(II) J Biol Chem 248:4229–4234
Fee JA, Gaber BP (1972) Anion binding to bovine erythrocyte superoxide dismutase. J Biol Chem 247:60–65
Forman JH, Evans HJ, Hill RL, Fridovich I (1973) Histidine at the active site of superoxide dismutase. Biochemistry 12:823–827
Fridovich I (1983) Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23:239–257
Fridovich I (1987) Superoxide dismutase. Adv Enzymol Relat Areas Mol Biol 58:61–97
Hore PJ (1983) A new method for water suppression in the proton NMR spectra of aqueous solutions. J Magn Reson 54:539–542
Horrocks Jr. W DeW, Greenburg ES (1971) Direct evaluation of dipolar nuclear magnetic resonance shifts from single crystal magnetic susceptibilities. Paramagnetic anisotropy of dichlorobis(triphenylphosphine) cobalt (II) and -nickel (II). Inorg Chem 10:2190–2194
Inubushi T, Becker ED, (1983) Efficient detection of paramagnetically shifted NMR resonances by optimizing the WEFT pulse sequence. J Magn Reson 51:128–133
Lippard SJ, Burge AR, Ugurbil K, Pantoliano MW, Valentine JS (1977) Nuclear magnetic resonance and chemical modification studies of bovine erythrocyte superoxide dismutase: evidence for zinc-promoted organization of the active site structure. Biochemistry 16:1136–1141
Marion D, Wutrich K (1983) Application of phase sensitive 2-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin constants in proteins. Biochem Biophys Res Commun 113:967–974
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymatic function for erytrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Mota de Freitas D, Ming LJ, Ramasamy R, Valentine JS (1990) 35Cl and 1H NMR study of anion binding to reduced bovine copper-zinc superoxide dismutase. Inorg Chem 29:3512–3518
Pantoliano MW, Valentine JS, Nafie LA (1982) Spectroscopic studies of copper(II) bound at the native copper site or substituted at the native zinc site of bovine erythrocuprein (superoxide dismutase) J Am Chem Soc 104:6310–6317
Ramaprasad S, Johnson RD, La Mar GN (1984) Vinyl mobility in myoglobin as studied by time-dependent nuclear Overhauser effect measurements. J Am Chem Soc 106:3632–3635
Sklenar V, Bax A. (1987) Spin-echo water suppression for the generation of pure-phase two-dimensional NMR spectra. J Magn Reson 74:469–479
Stoesz JD, Malinowski DP, Redfield AG (1979) Nuclear magnetic resonance study of solvent exchange and nuclear Overhauser effect of the histidine protons of bovine superoxide dismutase. Biochemistry 18:4669–4675
Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC (1982) Determination and analysis of the 2-Å structure of copper, zinc superoxide dismutase. J Mol Biol 160:181–217
Tainer JA, Getzoff ED, Richardson JS, Richardson DC (1983) Structure and mechanism of copper, zinc superoxide dismutase. Nature 306:284–287
Unger SW, LeComte JTJ, La Mar GN (1985) The utility of the nuclear Overhauser effect for peak assignment and structure elucidation in paramagnetic proteins. J Magn Reson 64:521–526
Valentine JS, Pantoliano MW (1982) Protein-metal ion interaction in cuprozinc protein (superoxide dismutase). In: Spiro TG (ed) Copper proteins, vol 3. Wiley, New York, pp 291–358
Author information
Authors and Affiliations
Additional information
Offprint requests to: I. Bertini
Rights and permissions
About this article
Cite this article
Bertini, I., Luchinat, B., Piccioli, M. et al. 1H NMR investigation of reduced copper-cobalt superoxide dismutase. Eur Biophys J 20, 269–279 (1991). https://doi.org/10.1007/BF00450562
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00450562