Summary
The oral bioavailability of methotrexate is variable and may be dose-dependent. The absorption of ‘interval’ oral methotrexate, which is given between cycles of chemotherapy, is unknown.
The bioavailability of oral methotrexate has been studied in eight patients, acting as their own controls, to assess the effect of subdivision of the dose, the formulation, and the timing of the methotrexate within the chemotherapy cycle.
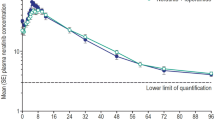
The mean bioavailability for all the oral methods of administration was 28.2%±3.7% compared with the same dose given IV. Absorption was uninfluenced by subdivision of the dose, liquid or tablet formulation, or administration on day 1 or day 10 of the chemotherapy cycle.
Similar content being viewed by others
References
Arnold AM, Williams CJ, Mead GM, Buchanan RB, Green JA Macbeth FM, Whitehouse JMA (1984) Combination chemotherapy using high-or low-dose methotrexate for small cell carcinoma of the lung — a randomised trial. Med Oncol Tumor Pharmacother 1: 9
Bell R, Sullivan JR, Moon WJ, Marty J, Shaw J (1978) Outpatient chemotherapy for breast cancer. Br Med J I: 857
Bleyer WA (1978) The clinical pharmacology of methotrexate: New applications for an old drug. Cancer 41: 36
Christophidis N, Vajdat JE, Lucas I, Moon JW, Louis WJ (1979) Comparison of intravenous and oral high-dose methotrexate in treatment of solid tumours Br Med J I: 298
Freeman MV (1958) The fluorimetric measurement of the absorption, distribution, and excretion of single doses of 4-amino-10-methyl-pteroylglutamic acid (amethopterin) in man. J Pharmacol Exp Ther 122: 154
Freeman MV, Narrod M, Gerstley BJ, Engstrom PF, Bornstein R (1975) Comparison of serum concentration of methotrexate after various routes of administration. Cancer 36: 1619
Frei E, Blum RH, Pitman SW, Kirkwood JM, Henderson IC, Skarin A, Maver RJ, Bast RC, Garnick MB, Parker LM, Canellos GP (1980) High-dose methotrexate with leucovorin rescue: Rationale and spectrum of antitumour activity. Am J Med 68: 370
Gushaw JB, Miller JG (1978) Homogenous enzyme immunoassay for methotrexate in serum. (Abstract) Clin Chem 24: 1032
Halprin KM, Fukui K, Okawarar A (1971) Blood levels of methotrexate and the treatment of psoriasis. Arch Dermatol 103: 243
Henderson ES, Adamson RM, Oliverio VT (1965) The metabolic fate of tritiated methotrexate. 11. Absorption and excretion in man. Cancer Res 25: 1018
Johnston A, Woollard RC (1983) STRIPE: An interactive computer program for the analysis of drug pharmacokinetics. J Pharmacol Methods 9: 193
Karnovsky DA, Burchenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM (ed) Evaluation of chemotherapeutic agents. Columbia Press, New York, p 191
Livingston RB, Carter SK (1970) Methotrexate In: Livingston RB, Carter SK (eds), Single Agents in Cancer Chemotherapy. IFI Plenum, New York, p 130
Noble WC, White PM, Baker H (1975) Assay of therapeutic doses of methotrexate in body fluids of patients with psoriasis. J Invest Dermatol 64: 69
Pinkerton CR, Welshman SG, Glasgow JFT, Bridges JM (1980) Can food influence the absorption of methotrexate in children with lymphoblastic leukaemia? Lancet II: 944
Pinkerton CR, Welshman SE, Kelly JG, Shanks RG, Bridges JM (1982) Pharmacokinetics of low-dose methotrexate in children receiving maintenance therapy for acute lymphoblastic leukaemia. Cancer Chemother Pharmacol 10: 36
Skarin AT, Canellos GP, Rosenthal DS, Case DC, Macintyre JM, Pinkus GS, Maloney WC, Frei E (1983) Improved prognosis of diffuse histiocytic and undifferentiated lymphoma by use of high-dose methotrexate alternating with standard agents (M-BA-COD). J Clin Oncol 1: 91
Smith DK, Omura GA, Ostroy F (1980) Clinical pharmacology of intermediate-dose oral methotrexate. Cancer Chemother Pharmacol 4: 117
Steele WJ, Stuart JFB, Lawrence JR, McNeill CA, Sneader WE, Whiting B, Calman KC, McVie JG (1979) Enhancement of methotrexate absorption by subdivision of dose. Cancer Chemother Pharmacol 3: 235
Stuart JFB, Calman KC, Watters J, Paxton J, Whiting B, Lawrence JR, Steele WH, McVie JG (1979) Bioavailability of methotrexate: Implications for clinical use. Cancer Chemother Pharmacol 3: 239
Wan SH, Huffman DH, Azarnoff DL, Stephens R, Hoogstraten B (1974) The effect of route of administration and effusions on methotrexate pharmacokinetics. Cancer Res 34: 3487
Zurek WS, Ojima Y, Anderson L, Collins GJ, Oberfields RA, Sullivan RD (1968) Pharmacologic studies of methotrexate in man. Obstet Gynaecol 126: 331
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harvey, V.J., Slevin, M.L., Woollard, R.C. et al. The bioavailability of oral intermediate-dose methotrexate. Cancer Chemother. Pharmacol. 13, 91–94 (1984). https://doi.org/10.1007/BF00257121
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00257121