Summary
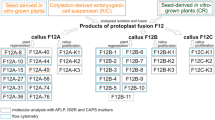
Mesophyll (M)- and suspension culture (S)-derived protoplasts of both Lycopersicon esculentum, tomato, and its wild relative Solanum lycopersicoides were fused as S+M, M+M and S+S combinations, respectively, to resolve the role of parental cell types in determining cpDNA transmission to intergeneric somatic hybrid plants. The mesophyll cpDNA was preferentially transmitted to 96% of the plants, each regenerated from a separate callus, in M+S and S+M fusion combinations. In contrast, for the M+M combination there was an equable distribution of either tomato cpDNA or that of S. lycopersicoides among the 34 hybrid plants. The number of plastids or proplastids in mesophyll or suspension protoplasts was not a factor regulating cpDNA transmission. Mesophyll or suspension protoplasts of both fusion partners had comparable frequencies of either plastid type with a mean of 23. The biased transmission of plastids from the mesophyll parent in somatic hybrid plants of S+M and M+S combinations appears to be due to differential multiplication of plastids, possibly conditioned by an unequal input of the nucleoids found in plastids versus proplastids. In the M+M fusion, plastid and nucleotid input and subsequent plastid multiplication are apparently equal, and when combined with random sorting out leads to an equal distribution of parental cpDNAs in the regenerated somatic hybrid plants. For the S+S combination, 22 somatic hybrid plants have exclusively tomato cpDNA, an outcome that is not readily explained by donor cell input.
Similar content being viewed by others
References
Akada S, Hirai A (1983) Plant Sci Lett 32:95–100
Butterfass T (1979) Patterns of chloroplast reproduction. Springer, Wien
Chen K, Wildman SG, Smith HH (1977) Proc Natl Acad Sci USA 74:5109–5112
Clark E, Schnabelrauch L, Hanson MR, Sink KC (1986) Theor Appl Genet 72:748–755
Coleman AW (1984) Exp Cell Res 152:528–540
Corriveau JL, Polans NO, Coleman AW (1989) Curr Genet 16:47–51
Cseplo A, Nagy F, Maliga P (1984) Mol Gen Genet 198:7–11
Flick CE, Kut SA, Bravo JE, Gleba YY, Evans DA (1985) Bio/ Technology 3:555–560
Fluhr R, Aviv D, Galun E, Edelman M (1984) Theor Appl Genet 67:491–497
Frearson EM, Power JB, Cocking EC (1973) Dev Biol 33:130–137
Gleba YY, Kolesnik NN, Meshkene IV, Cherep NN, Parokonny AS (1984) Theor Appl Genet 69:121–128
Guri A, Sink KC (1988) Theor Appl Genet 76:490–496
Handley LW, Sink KC (1985) Plant Sci 42:201–207
Handley LW, Nickels RL, Cameron MW, Moore PP, Sink KC (1986) Theor Appl Genet 71:691–697
Herrmann RG, Kowallik KV (1970) Protoplasma 68:365–372
Levi A, Ridley BL, Sink KC (1988) Curr Genet 14:177–182
James TW, Jope C (1978) J Cell Biol 79:623–630
Kowallik KV, Herrmann RG (1972) J Cell Sci 11:357–377
Kumar A, Cocking EC (1987) Am J Bot 74:1289–1303
Kuroiwa TS, Suzuki T, Ogawa K, Kawano S (1981) Plant Cell Physiol 22:381–396
Maniatis T, Fritzsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual cloning. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Miyamura S, Nagata T, Kuroiwa T (1986) Protoplasma 133:66–72
Miyamura S, Kuroiwa T, Nagata T (1987) Protoplasma 141:149–159
Murashige T, Skoog F (1962) Physiol Plant 15:473–497
Niedz RP, Rutter SM, Handley LW, Sink KC (1985) Plant Sci 39:199–204
Palmer JD (1986) Methods Enzymol 118:167–186
Pehu E, Karp A, Moore K, Steele S, Dunckley R, Jones MGK (1989) Theor Appl Genet 78:696–704
Phillips AL (1985) Curr Genet 10:147–152
Possingham JV (1980) Annu Rev Plant Physiol 31:113–129
Possingham JV, Smith JW (1972) J Exp Bot 23:1050–1059
Rogers SO, Bendich AJ (1988) Plant Mol Biol Manual A 6:1–10
Rose RJ, Thomas MR, Filler JT (1990) Aust J Plant Physiol 17:303–321
Scandalios JG (1969) Biochem Genet 3:37–39
Scott NS, Tymms MJ, Possingham JV (1984) Planta 161:12–19
Scowcroft WR, Larkin PJ (1981) Theor Appl Genet 60:179–184
Thomas MR, Rose RJ (1983) Planta 158:329–338
Yasuda T, Kuroiwa T, Nagata T (1988) Planta 174:235–241
Author information
Authors and Affiliations
Additional information
Communicated by C. S. Levings III
Rights and permissions
About this article
Cite this article
Li, Y., Sink, K.C. Cell type determines plastid transmission in tomato intergeneric somatic hybrids. Curr Genet 22, 167–171 (1992). https://doi.org/10.1007/BF00351478
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00351478