Summary
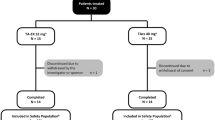
The safety profile of a new sustained-release (SR) form of etodolac was evaluated in 539 young and elderly patients with osteoarthritis or rheumatoid arthritis. Four long-term, open-label studies were conducted in nine different countries totaling 3,827 patient-months' exposure to etodolac SR. Patients were treated with either 400 mg or 600 mg etodolac SR once a day for up to 52 weeks. Withdrawals due to adverse reactions were low, occurring in only 5% (26/539) of all patients. The most common drug-related study events were GI-related, occurring in <8% of patients. Elderly persons (≥65 years of age) were not at greater risk for adverse reactions or drug-related study events than were younger patients. Serious GI-related study events were rare (0.2%). The low level of serious GI effects was consistent with a separate study measuring gastrointestinal (GI) blood loss. Etodolac SR produced significantly less GI blood loss than naproxen in normal subjects. Because of its favorable safety profile, etodolac SR can serve as an alternative to conventional etodolac, providing the convenience of once-daily administration.
Similar content being viewed by others
References
Serni U (1990) Global safety of etodolac: reports from world-wide postmarketing surveillance studies. Rheumatol Int 10:23–27
Karbowski A (1989) A global safety evaluation of etodolac. Clin Rheumatol 8:73–79
Benhamou CL (1990) Large-scale open trials with etodolac (Lodine®) in France: an assessment of safety. Rheumatol Int 10:29–34
Schattenkirchner M (1990) An updated safety profile of etodolac in several thousand patients. Eur J Rheumatol Inflamm 10:56–65
Arnold JD, Mullane JF, Hayden DM, March L, Hart K, Perdomo CA, Fencik M, Berger AE (1984) Etodolac, aspirin, and gastrointestinal microbleeding. Clin Pharmacol Ther 35:716–721
Arnold JD, Salom IL, Berger AE, Meinders JD, Jacob G, Hayden D, Mullane JF (1985) Comparison of gastrointestinal microbleeding associated with use of etodolac, ibuprofen, indomethacin, and naproxen in normal subjects. Curr Ther Res 37:730–738
Salom I, Jacob G, Jallad N, Perdomo CA, Mullane JF, Weidler D (1984) Gastrointestinal microbleeding associated with the use of etodolac, ibuprofen, indomethacin and naproxen in normal males. J Clin Pharmacol 24:240–246
Jallad NS, Sanda M, Salom IL, Perdomo CS, Garg DC, Mullane JF, Weidler DJ (1986) Gastrointestinal blood loss in arthritic patients receiving chronic dosing with etodolac and piroxicam. Am J Med Sci 292:272–276
Lanza F, Panagides J, Salom IL (1986) Etodolac compared with aspirin: an endoscopic study of the gastrointestinal tracts of normal volunteers. J Rheumtol 13:299–303
Lanza F, Rack MF, Lynn M, Wolf J, Sanda M (1987) An endoscopic comparison of the effects of etodolac, indomethacin, ibuprofen, naproxen, and placebo on the gastrointestinal mucosa. J Rheumatol 14:338–341
Bianchi Porro G, Caruso I, Petrillo M et al. (1991) A double-blind gastroscopic evaluation of the effects of etodolac and naproxen on the gastrointestinal mucosa of rheumatic patients. J Intern Med 229:5–8
Russell RI (1990) Endoscopic evaluation of etodolac and naproxen, and their relative effects on gastric and duodenal prostaglandins. Rheumatol Int 10:17–21
Green JA (1984) Indomethacin sustained-release? Drug Intell Clin Pharm 18:1004–1007
Yeh KC (1985) Pharmacokinetic overview of indomethacin and sustained release indomethacin. Am J Med 79:3–12
Kaarela K, Lehtinen K, Makisara P (1982) Pharmacokinetics and tolerance of slow release indomethacin tablets in rheumatoid arthritis. Eur J Clin Pharmacol 23:349–351
Houghton GW, Dennis MJ, Rigler ED (1984) Comparative pharmacokinetics of ketoprofen derived from single oral doses of ketoprofen capsules or a renal sustained-release pellet formulation. Biopharm Drug Dispos 5:203–209
Marcolongo R, Rubegni M, Provvedi D, et al. (1984) A double-blind interpatient comparison of plain and slow-release ketoprofen in osteoarthritis. Int J Clin Pharmacol Ther Toxicol 22:377–381
Schubiger BI, Ciccolunghi SN, Tanner K (1980) Once daily dose treatment with non-steroidal antirheumatic drug (diclofenac) in osteoarthrosis. J Int Med Res 8:167–174
Dreiser RL (1993) Efficacy of etodolac SR (this suppl)
Leese P (1992) Comparison of the effects of etodolac SR and naproxen on gastro-intestinal blood loss. Curr Med Res Opin 13:13–20
Benet L (1993) EULAR paper (bioequivalence) (this suppl)
Jansen PAF, Van Ginneken CAM, Gribnau FWJ (1987) Is dose adjustment of nonsteroidal anti-inflammatory drugs necessary in the elderly? A review of the pharmacokinetics of NSAID in the aged. Neth J Med 30:248–258
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schattenkirchner, M. The safety profile of sustained-release etodolac. Rheumatol Int 13 (Suppl 2), S31–S35 (1993). https://doi.org/10.1007/BF00290282
Issue Date:
DOI: https://doi.org/10.1007/BF00290282