Summary
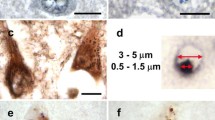
An immunocytochemical study of Alzheimer's disease hippocampus with a panel of anti-tau antibodies revealed two antibodies that stained granulovacuolar bodies (GVB) in pyramidal neurons of Ammon's horn. These two affinity-purified anti-tau antibodies were raised in rabbits against synthetic peptides homologous to sequences (amino acids 44–55 and 75–87) in the 58 amino acid insert in the amino terminus of the longest form of human tau. This region is homologous to exons 2 and exon 3 of bovine tau. The exon 2 peptide contains a serine (amino acid residue 46), which has been shown to be a phosphorylated site in paired helical filaments. Antibodies to a nonphosphorylated exon 2 peptide failed to immunostain GVB, but those to the phosphopeptide consistently stained GVB. Staining, however, was most consistent with the antibody to the exon 3 sequence. As in previous studies, GVB were also stained by RT97, a neurofilament antibody whose epitope in tau appears to be a phosphorylated site in or near exon 2, perhaps at serine residue 46 (Brion et al. 1992). Antibodies to epitopes in the amino terminus, mid-region and carboxy terminus of tau failed to consistently stain GVB. More often they produced staining around the periphery of the GVB, giving the appearance of an “empty vacuole.” Most GVB were also immunoreactive with an antibody to ubiquitin. The results are consistent with the hypothesis that GVB are derived from sequestered altered tau possibly mediated by ubiquitin. The failure to detect most regions of tau in GVB is consistent with the idea that tau is partially degraded or highly modified in GVB.
Similar content being viewed by others
References
Ball MJ (1978) Topographic distribution of neurofibrillary tangles and granulovacuolar degeneration in hippocampal cortex of aging and demented patients. A quantitative study. Acta Neuropathol (Berl) 42:73–80
Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res 477:90–99
Binder LI, Frankfurter A, Rebhun L (1985) The distribution of tau polypeptides in the mammalian central nervous system. J Cell Biol 101:1371–1378
Bondareff W, Wischik CM, Novak M, Roth M (1991) Sequestration of tau by granulovacuolar degeneration in Alzheimer's disease. Am J Pathol 139:641–647
Brion JP, Couck AM, Anderton BH (1992) Isolated PHF and PHF-tau (A68) polypeptides in Alzheimer's disease contain epitopes from the whole sequence of different tau isoforms and modified sites recognized by RT97 and 8D8 anti-neurofilament antibodies. Neurobiol Aging 18 [Suppl]:S54–55
Crowe A, Ksiezak-Reding H, Liu W-K, Dickson DW, Yen S-H (1991) The N-terminal region of human tau is present in Alzheimer disease protein A68 and is incorporated into paired helical filaments. Am J Pathol 139:1463–1470
Dickson DW, Ksiezak-Reding H, Crowe A, Yen S-H (1987) Monoclonal antibodies show cross reactivity of Alzheimer neurofibrillary tangles and heat-stable microtubule-associated proteins. Adv Behav Biol 34:165–180
Dickson DW, Ksiezak-Reding H, Davies P, Yen S-H (1987) A monoclonal antibody that recognizes a phosphorylated epitope in Alzheimer neurofibrillary tangles, neurofilaments and tau proteins immunostains granulovacuolar degeneration. Acta Neuropathol (Berl) 73:254–258
Dickson DW, Ksiezak-Reding K, Liu W-K, Davies P, Crowe A, Yen S-H (1992) Immunocytochemistry of neurofibrillary tangles with antibodies to subregions tau of protein: identification of hidden and cleaved epitopes and a new phosphorylation site. Acta Neuropathol 84:596–605
Dickson DW, Wertkin A, Kress Y, Ksiezak-Reding H, Yen S-H (1990) Ubiquitin immunoreactive structures in normal human brains. Distribution and development aspects. Lab Invest 63:87–99
Drewes G, Lichtenberg-Kraag B, Doring F, Mandelkow E-M, Biernat J, Goris J, Doree M, Mandelkow E (1992) Mitogen activated protein (MAP) kinase transforms tau protein into and Alzheimer-like state. EMBO J 11:2131–2138
Finley D, Chau V (1991) Ubiquitination. Annu Rev Cell Biol 7:25–69
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3:519–526
Goedert M, Spillantini MG, Jakes R (1991) Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau. Neurosci Lett 126:149–154
Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein τ (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917
Hirano A, Dembitzer HM, Kurland LY (1968) The fine structure of some intraganglionic alterations. Neurofibrillary tangles, granulovacuolar bodies and “rod-like” structures as seen in Guam amyotrophic lateral sclerosis and Parkinson's dementia complex. J Neuropathol Exp Neurol 67:167–182
Hyman BT, Van Hoesen GW, Wolozin BL, Davies P, Kromer LJ, Damasio AR (1988) Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol 23:371–379
Kahn J, Anderton BH, Probst A, Ulrich J, Esiri MM (1985) Immunohistological study of granulovacuolar degeneration using monoclonal antibodies to neurofilaments. J Neurol Neurosurg Psychiatry 48:924–926
Ksiezak-Reding H, Yen S-H (1991) Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron 6:717–728
Ksiezak-Reding H, Dickson DW, Davies P, Yen S-H (1987) Recognition of tau epitopes by anti-neurofilament antibodies that stain Alzheimer neurofibrillary tangles. Proc Natl Acad Sci USA 84:3410–3414
Lee VM-Y, Balin BJ, Otvos L, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 231:675–678
Liu WS, Dickson DW, Yen S-H (1993) Heterogeneity of tau protein in Alzheimer's disease: evidence for increased expression of an isoform and preferential distribution of a phosphorylated isoform in neurites. Am J Pathol 142:387–394
Lubke U, Mercken M, Vandemeeren M, Ceuterick C, Van Mechelen E, Martin J-J, Cras P (1992) Comparative study of granulovacuolar degeneration in neurodegenerative diseases. Neurobiol Aging 18 [Suppl]:S41
Mann DMA (1978) Granulovacuolar degeneration in pyramidal cells of the hippocampus. Acta Neuropathol (Berl) 42:149–151
Okamoto K, Hirai S, Iizuka T, Yanagisawa T, Watanabe M (1991) Reexamination of granulovacuolar degeneration. Acta Neuropathol 82:340–345
Okamoto K, Hirai S, Yamaguchi H, Ishiguro K, Takatama M (1992) Immunohistochemical and ultrastructural studies of granulovacuolar degeneration. Neurobiol Aging 18 [Suppl.]:S41
Oyanagi S, Ikuta F (1974) An ultrastructural observation on granulovacuolar degenerations in reference to the process of their formation. Brain Nerve (Tokyo) 26:783–788
Price DL, Altschuler RJ, Struble RG, Casanova MF, Cork LC, Murphy DB (1986) Sequestration of tubulin in neurons in Alzheimer's disease. Brain Res 385:305–310
Sternberger LA, Sternberger NH (1982) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA 80:6126–6130
Terry RD, Wisniewski HM, Johnson AB (1970) Studies on the formation of autophagic vacuoles in neurons treated with spindle inhibitors (colchicine and vinblastine) (abstract). J Neuropathol Exp Neurol 29:142–143
Tomlinson BE, Kitchener D (1972) Granulovacuolar degeneration of hippocampal pyramidal cells. J Pathol 106:165–185
Wolozin BL, Pruchnicki A, Dickson DW, Davies P (1986) A neuronal antigen in the Alzheimer brain. Science 232:648–650
Author information
Authors and Affiliations
Additional information
Supported by: AG60803, AG01136 and AG04145
Rights and permissions
About this article
Cite this article
Dickson, D.W., Liu, W.K., Kress, Y. et al. Phosphorylated tau immunoreactivity of granulovacuolar bodies (GVB) of Alzheimer's disease: localization of two amino terminal tau epitopes in GVB. Acta Neuropathol 85, 463–470 (1993). https://doi.org/10.1007/BF00230483
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230483