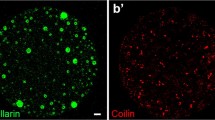
Abstract
The sphere organelles (spheres) ofXenopus and other amphibian oocytes are known to contain small nuclear ribonucleoprotein particles (snRNPs) and have been suggested to play a role in snRNP complex assembly. Coupled with the similarities that exist between spheres and nucleoli and the quantitative and kinetic aspects of snRNA synthesis in theXenopus oocyte, we have investigated whether or not the U snRNA encoding genes are amplified inXenopus oogenesis, the spheres being possible sites for the location of such extrachromosomal gene copies. By applying a number of quantitative nucleic acid hybridization procedures to both total and fractionated oocyte and somatic DNA, employing both homologous and heterologous U snRNA gene probes and suitable amplification and non-amplification control probes, we show that the U snRNA genes do not undergo any major amplification inXenopus oogenesis. Therefore, the analogy between the sphere organelles and nucleoli appears to be limited. The role of the spheres and their relationship to other snRNP containing structures, specifically B snurposomes, and the sphere organizer loci remains obscure.
Similar content being viewed by others
References
Bird AP (1980) Gene reiteration and gene amplification. Cell Biol 3:111–362
Bird AP, Birnstiel ML (1971) A timing study of gene amplification inXenopus laevis oocytes. Chromosoma 35:300–309
Boseley P, Moss T, Mächler M, Portmann R, Birnstiel ML (1979) Sequence organization of the spacer DNA in a ribosomal gene unit ofXenopus laevis. Cell 17:19–31
Brown DD, Dawid IB (1968) Specific gene amplification in oocytes. Science 160:272–280
Brown DD, Weber CS (1968) Gene linkage by RNA-DNA bybridization: I. Unique DNA sequences homologous to 4S RNA, 5S RNA and ribosomal RNA. J Mol Biol 34:661–680
Carmo-Fonseca M, Mollervey D, Barabino SML, Merdes A, Brunner C, Zamore PD, Green MR, Hurt E, Lamond AI (1990) Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J 10:195–206
Carroll D, Brown DD (1976) Repeating units ofXenopus laevis oocyte-type 5S DNA are heterogeneous in length. Cell 7:467–475
Ciliberto G, Buckland R, Cortese R, Philipson L (1985) Transcription signals in embryonicXenopus laevis U1 RNA genes. EMBO J 4:1537–1543
Dawid IB·(1965) Deoxyribonucleic acid in amphibian eggs. J Mol Biol 12:581–599
Dawid IB, Brown DD (1970) The mitochondrial and ribosomal DNA components of oocytes ofUrechis caupo. Dev Biol 22:1–14
Dawid IB, Brown DD, Reeder R (1970) Composition and structure of chromosomal and amplified ribosomal DNAs ofXenopus laevis. J Mol Biol 51:341–360
Evans D, Brinstiel ML (1968) Localization of amplified ribosomal DNA in the oocyte ofXenopus laevis. Biochim Biophys Acta 166:274–276
Fedoroff NV, Brown DD (1978) The nucleotide sequence of oocyte 5S ribosomal DNA inXenopus laevis. I. The AT-rich spacer. Cell 13:701–716
Feinberg AP, Vogelstein B (1983) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Forbes DJ, Kornberg TB, Kirschner MW (1983) Small nuclear RNA transcription and ribonucleoprotein assembly in early development. J Cell Biol 97:62–72
Fritz A, Parisot R, Newmeyer D, DeRobertis EM (1984) Small nuclear U-ribonucleoproteins in development: uncoupled accumulation of the protein and RNA components. J Mol Biol 178:273–285
Gall JG (1968) Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc Natl Acad Sci USA 60:553–560
Gall JG (1992) Organelle assembly and function in the amphibian germinal vesicle. Adv Dev Biochem (in press)
Gall JG, Callan HG (1989) The sphere organelle contains small nuclear ribonucleoproteins. Proc Natl Acad Sci USA 86:6635–6639
Gall JG, Stephenson EC, Erba HP, Diaz MO, Barsacchi-Pilone G (1981) Histone genes are located at the sphere loci of newt lampbrush chromosome. Chromosoma 84:159–171
Georgiev O, Mous J, Birnstiel ML (1984) Processing and nucleocytoplasmic transport of histone gene transcripts. Nucleic Acids Res 12:8539–8551
Kay BK, Dawid IB (1983) The 1723 element: A long, homogeneous, highly repeated DNA unit interspersed in the genome ofXenopus laevis. J Mol Biol 170:583–596
Kazmaier M, Tebb G, Mattaj IW (1987) Functional characterization ofX. laevis U5 snRNA genes. EMBO J 6:3071–3078
Kidder GM (1976) The ribosomal cistrons in clam gametes. Dev Biol 49:132–142
Krol A, Lund E, Dahlberg JE (1985) Two embryonic U1 RNA genes ofXenopus laevis have both common and gene-specific transcription signals. EMBO J 4:1529–1535
Lund E, Dahlberg JE, Forbes DJ (1984) The two embryonic U1 small nuclear RNAs ofXenopus laevis are encoded by a major family of tandemly repeated genes. Mol Cell Biol 4:2580–2586
Mattaj IW, Zeller R (1983)Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5′ and 3′ flanking homology with other RNA polymerase H transcribed genes. EMBO J 2:1883–1891
Mattaj IW, Zeller R, Carrasco AE, Jamrich M, Lienhard S, DeRobertis EM (1985) U snRNA gene families inXenopus laevis. In: Maclean N (ed) Oxford surveys of eukaryotic genes, vol 2. Oxford University Press, Oxford, pp 121–140
Miller JR, Cartwright EM, Brownlee GG, Fedoroff NV, Brown DD (1978) The nucleotide sequence of oocyte 5S ribosomal DNA inXenopus laevis. II. The GC-rich region. Cell 13:717–725
Perkowska E, Macgregor HC, Birnstiel ML (1968) Gene amplification in the oocyte nucleus of mutant and wild-typeXenopus laevis. Nature 217:649–650
Reddy R, Busch H (1988) Small nuclear RNAs: RNA sequences, structure and modifications. In: Birnstiel ML (ed) Structure and function of major and minor small nuclear ribonucleoprotein particles. Springer, Berlin Heidelberg New York, pp 1–37
Reddy R, Henning D, Busch H (1985) Primary and secondary structure of U8 small nuclear RNA. J Biol Chem 260:10930–10935
Rochaix JB, Bird AP, Bakken A (1974) Ribosomal RNA gene amplification by rolling circles. J Mol Biol 87:473–487
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Wallace H, Birnstiel ML (1966) Ribosomal cistrons and the nucleolar organizer. Biochim Biophys Acta 114:296–310
Watson-Coggins L, Gall JG (1972) The timing of meiosis and DNA synthesis during early oogenesis in the toad,Xenopus laevis. J Cell Biol 52:569–576
Wu Z, Murphy C, Callan HG, Gall JG (1991) Small nuclear ribonucleoproteins and heterogeneous nuclear ribonucleoproteins in the amphibian germinal vesicle: loops, spheres and snurposomes. J Cell Biol 113:465–483
Zeller R, Carri M-T, Mattaj IW, DeRobertis EM (1984)Xenopus laevis U1 snRNA genes: characterization of transcriptionally active genes reveals major and minor repeated gene families. EMBO J 3:1075–1081
Author information
Authors and Affiliations
Additional information
by A. Spradling
Rights and permissions
About this article
Cite this article
Phillips, S., Cotten, M., Laengle-Rouault, F. et al. Amphibian oocytes and sphere organelles: are the U snRNA genes amplified?. Chromosoma 101, 549–556 (1992). https://doi.org/10.1007/BF00660314
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00660314