Summary
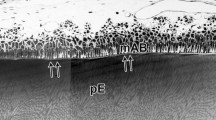
Enamel proteins were extracted from the newly formed layer of immature porcine enamel, and the 25 kDa amelogenin, 89 kDa enamelin and 13–17 kDa nonamelogenins were purified. Specific antisera were raised against these proteins. Antibodies specific to the C-terminal region (residues 149–173) of the 25 kDa amelogenin were generated by absorption of the anti-25 kDa amelogenin serum with 20 kDa amelogenin, which contains residues 1–148 of the antigen. Immunoelectrotransfer blotting of the extracted porcine enamel proteins showed that the anti-25 kDa amelogenin serum recognized the 25 kDa and other low and high molecular weight amelogenins. The C-terminal specific anti-25 kDa amelogenin serum reacted only with amelogenins having molecular weights over 23 kDa. The anti-89 kDa enamelin serum recognized the 89 kDa enamelin and lower molecular weight proteins, but neither the amelogenins nor the 13–17 kDa nonamelogenins. The antiserum against the 13–17 kDa nonamelogenins showed no cross reactivity to the 89 kDa enamelin, but recognized higher molecular weight nonamelogenins. In immunohistochemical preparations of the porcine tooth germs, the 25 kDa amelogenin-like immunoreactivity over immature enamel decreased in a gradient from the enamel surface to the middle layer. In the inner layer immunoreactivity was concentrated over the prism sheaths. The C-terminal specific 25 kDa amelogenin-like immunoreactivity was intense at the outer layer of immature enamel and decreased sharply toward the middle layer. Prism sheaths were intensely stained by the antiserum to the 13–17 kDa nonamelogenins. The 89 kDa enamelin-like immunoreactivity over enamel prisms was intense at the outer layer and decreased toward the middle layer. Staining by the anti-89 kDa enamelin serum of prism sheaths was faint. In immature rat incisor enamel, the C-terminal specific 25 kDa amelogenin antiserum demonstrated a staining pattern similar to that in the immature enamel of the pig. Distinct 13–17 kDa nonamelogenin-like and 89 kDa enamelin-like immunoreactivities were found especially in the layer adjacent to the Tomes' process. We conclude that some enamel proteins are degraded soon after their secretion from the secretory ameloblast in the rat and the pig. The specific enamel proteins which reacted with the antiserum to the 13–17 kDa nonamelogenins seem to be involved with the formation of prism sheaths in immature porcine enamel, but not in rat incisor enamel.
Similar content being viewed by others
References
Aoba T, Tanabe T, Moreno EC (1987) Functions of amelogenins in porcine enamel mineralization during the secretory stage of amelogenesis. Adv Dent Res 1:252–260
Burgess RC, Maclaren CM (1965) Proteins in developing bovine enamel. In: Stack MV, Fearnhead RW (eds) Tooth enamel. John Wright and Sons, Bristol, pp 74–82
Danscher G, Nörgaard JOR (1983) Light microscopic visualization of colloidal gold on resin-embedded tissue. J Histochem Cytochem 31:1394–1398
De Mey J (1983) Colloidal gold probes in immunocytochemistry. In: Polak JM, Van Noorden S (eds) Immunocytochemistry. Practical applications in pathology and biology. John Wright and Sons, Bristol, pp 82–112
Eastoe JE (1979) Enamel protein chemistry — past, present and future. J Dent Res 58(B):753–763
Fukae M, Shimizu M (1974) Studies on the proteins of developing bovine enamel. Arch Oral Biol 19:381–386
Fukae M, Tanabe T (1985) Separation of a non-amelogenin component from purified amelogenin preparation of immature porcine enamel. Jpn J Oral Biol 27:1249–1251
Fukae M, Tanabe T (1987) Nonamelogenin components of porcine enamel in the protein fraction free from the enamel crystals. Calcif Tissue Int 40:286–293
Fukae M, Tanabe T, Shimizu M (1977) Proteolytic enzyme activity in porcine immature enamel. Tsurumi Univ Dent J 3:15–17
Fukae M, Tanabe T, Ijiri H, Shimizu M (1980) Studies on porcine enamel proteins: a possible original enamel protein. Tsurumi Univ Dent J 6:87–94
Glimcher MJ, Travis DF, Friberg UA, Mechanic GL (1964) The electron microscopic localization of the neutral soluble proteins of developing bovine enamel. J Ultrastruct Res 10:362–376
Graver HT, Herold RC, Chung T-Y, Christner PJ, Pappas C, Rosenbloom J (1978) Immunofluorescent localization of amelogenins in developing bovine teeth. Dev Biol 63:390–401
Herold RC, Rosenbloom J (1990) Immunocytochemical localization of enamelin proteins in developing bovine teeth. Arch Oral Biol 35:373–379
Herold RC, Graver HT, Christner P (1980) Immunohistochemical localization of amelogenins in enameloid of lower vertebrate teeth. Science 207:1357–1358
Herold RC, Boyde A, Rosenbloom J, Lally ET (1987) Monoclonal antibody and immunogold cytochemical localization of amelogenins in bovine secretory amelogenesis. Arch Oral Biol 32:439–444
Holgate CS, Jackson P, Cowen PN, Bird CC (1983) Immunogoldsilver staining: new method of immunostaining with enhanced sensitivity. J Histochem Cytochem 31:938–944
Inage T, Shimokawa H, Teranishi Y, Iwase T, Toda Y, Moro I (1989) Immunocytochemical demonstration of amelogenins and enamelins secreted by ameloblasts during the secretory and maturation stages. Arch Histol Cytol 52:213–229
Kallenbach E (1977) Fine structure of secretory ameloblasts in the kitten. Am J Anat 148:479–512
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leblond CP, Warshawsky H (1979) Dynamics of enamel formation in the rat incisor tooth. J Dental Res 58(B):950–975
Limeback H, Simic A (1989) Porcine high molecular weight enamel proteins are primarily stable amelogenin aggregates and serum albumin-derived proteins. In: Fearnhead RW (ed) Tooth enamel, V. Florence Publishers, Yokohama, pp 269–277
Nanci A, Bendayan M, Slavkin HC (1985) Enamel protein biosynthesis and secretion in mouse incisor secretory ameloblasts as revealed by high-resolution immunocytochemistry. J Histochem Cytochem 33:1153–1160
Ogata Y, Shimokawa H, Sasaki S (1988) Purification, characterization, and biosynthesis of bovine enamelins. Calcif Tissue Int 43:389–399
Rosenbloom J, Lally E, Dixon M, Spencer A, Herold R (1986) Production of a monoclonal antibody to enamelins which does not cross-react with amelogenins. Calcif Tissue Int 39:412–415
Roth J, Taatjes DJ, Warhol MJ (1989) Prevention of non-specific interactions of gold labeled reagents on tissue sections. Histochemistry 92:47–56
Shimizu M, Fukae M (1983) Enamel proteins. In: Suga S (ed) Mechanism of tooth enamel formation. Quintessence Publishing, Tokyo, pp 125–141
Shimizu M, Tanabe T, Fukae M (1979) Proteolytic enzyme in porcine immature enamel. J Dent Res 58(B):782–789
Shimokawa H, Ogata Y, Sasaki S, Sobel ME, McQuillan CI, Termine JD, Young MF (1987) Molecular cloning of bovine amelogenin cDNA. Adv Dent Res 1:293–297
Shimokawa H, Tamura M, Ibaraki K, Ogata Y, Sasaki S (1989) Human amelogenin gene. In: Fearnhead RW (ed) Tooth enamel, V. Florence Publishers, Yokohama, pp 301–307
Sicher H (1962) Orban's oral histology and embryology, 5th edn. Mosby, Saint Louis, pp 55–61
Slavkin HC, Zeichner-David M, MacDougall M, Bringas P, Bessem C, Honig LS (1982) Antibodies to murine amelogenins: localization of enamel proteins during tooth organ development in vitro. Differentiation 23:73–82
Slavkin HC, Bessem C, Bringas P, Zeichner-David M, Nanci A, Snead ML (1988) Sequential expression and differential function of multiple enamel proteins during fetal, neonatal, and early postnatal stages of mouse molar amelogenesis. Differentiation 37:26–39
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38:87–93
Smith CE, Pompura JR, Borenstein S, Fazel A, Nanci A (1989) Degradation and loss of matrix proteins from developing enamel. Anat Rec 224:292–316
Snead ML, Lau EC, Zeichner-David M, Fincham AG, Woo SLC, Slavkin HC (1985) DNA sequence for cloned cDNA for murine amelogenin reveal the amino acid sequence for enamel-specific protein. Biochem Biophys Res Commun 129:812–818
Tanabe T (1984) Purification and characterization of proteolytic enzyme in porcine immature enamel (in Japanese with English summary). Tsurumi U Dent J 10:443–452
Termine JD, Belcourt AB, Christner PJ, Conn KM, Nylen MU (1980) Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem 255:9760–9768
Towbin H, Staehelin T, Gordon J (1974) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Travis DF, Glimcher MJ (1964) The structure and organization of, and the relationship between the organic matrix and the in organic crystals of embryonic bovine enamel. J Cell Biol 23:447–497
Uchida T (1985) Serotonin-like immunoreactivity in the taste bud of the mouse circumvallate papilla (in Japanese with English abstract). Jpn J Oral Biol 27:132–139
Uchida T, Fukae M, Tanabe T (1989) Immunocytochemical localization of amelogenins in the deciduous tooth germs of the human fetus. Arch Histol Cytol 52:543–552
Wakita M, Tsuchiya H, Gunji T, Kobayashi S (1981) Three-dimensional structure of Tomes' processes and enamel prism formation in the kitten. Arch Histol Jpn 44:285–297
Yamakoshi Y, Tanabe T, Fukae M, Shimizu M (1989) Amino acid sequence of porcine 25 kDa amelogenin. In: Fearnhead RW (ed) Tooth enamel, V. Florence Publishers, Yokohama, pp 314–321
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uchida, T., Tanabe, T., Fukae, M. et al. Immunochemical and immunohistochemical studies, using antisera against porcine 25 kDa amelogenin, 89 kDa enamelin and the 13–17 kDa nonamelogenins, on immature enamel of the pig and rat. Histochemistry 96, 129–138 (1991). https://doi.org/10.1007/BF00315983
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315983