Summary
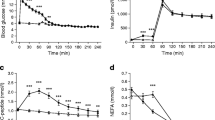
The effect of hyperglycaemia on hepatic glucose production (R a ) was investigated in nine healthy men using sequential clamp protocols during somatostatin infusion and euglycaemia (0–150 min), at plasma glucose levels of 165 mg · dl−1 (9.2 mM, 150–270 min) and during insulin infusion (1.0 mU · kg−1 · min−1, 270–360 min) in study 1 or during hypo-insulinaemia and plasma glucose levels of 220 mg · dl−1 (12.2 mM; 270–390 min) in study 2. Somatostatin decreased R a and glucose disposal rate (R d ) but increased plasma free fatty acids (FFA) and lipid oxidation during euglycaemia. Increasing plasma glucose to 165 mg · dl−1 (9.2 mM) and hypo-insulinaemia increased R d , but no suppressive effects on R a , plasma FFA and lipid oxidation were observed. By contrast hyperinsulinaemia (study 1), as well as a further increase in plasma glucose (study 2), both decreased R a . However, more pronounced hyperglycaemia increased insulin secretion despite somatostatin resulting in a fall in plasma FFA and lipid oxidation. Our data questions the accepted dogma that hyperglycaemia inhibits R a independently of insulin action.
Similar content being viewed by others
References
Adkins BA, Myers SR, Hendrick GK, Stevenson RW, Williams PE, Cherrington AD (1987) Importance of route of intravenous glucose delivery to hepatic glucose balance in conscious dog. J Clin Invest 9:557–565
Baron AD, Kolterman OG, Bell J, Mandarino LJ, Olefsky JM (1985) Rates of noninsulin-mediated glucose uptake are elevated in type 2 diabetic subjects. J Clin Invest 76:1782–1788
Bell PM, Firth RG, Rizza RA (1986a) Effects of hyperglycemia on glucose production and utilization in humans: measurement with (23H)-, (33H)- and (614C) glucose. Diabetes 35:642–648
Bell PM, Firth RG, Rizza RA (1986b) Assessment of insulin action in insulin-dependent diabetes mellitus using (614C)glucose, (33H)glucose, and (23H)glucose. Differences in the apparent pattern of insulin resistance depending on the isotope used. J Clin Invest 78:1479–1486
Bergman RN, Finegood DT, Ader M (1985) Assessment of insulin sensitivity in vivo. Endocr Rev 6:45–86
Christin L, Nacht CA, Vernet O, Ravussin E, Jequier E, Acheson KJ (1986) Insulin. Its role in the thermic effect of glucose. J Clin Invest 77:1747–1755
Cobelli C, Mari A, Ferrannini E (1987) Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol 252:E679-E689
Davidson MB (1981) Autoregulation by glucose of hepatic glucose balance: permissive effect of insulin. Metabolism 30:279–284
DeFronzo RA (1988) The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687
DeFronzo RA, Ferrannini E, Hendler R, Felig R, Wahren J (1983) Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycaemia. Diabetes 32:35–45
Ferrannini E, Del Prato S, DeFronzo RA (1986) Glucose kinetics tracer methods. In: Clark WL, Larner J (eds) Methods in diabetes research. Wiley, New York, pp 108–141
Gray RS, Scarlett JA, Griffin J, Olefsky JM, Kolterman OG (1982) In vivo deactivation of peripheral, hepatic and pancreatic insulin action in man. Diabetes 31:929–936
Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA (1989) Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84:205–213
Jackson RA, Hamling JB, Sim BM, Blix PM, Jaspan JB, Markanday S, Nabarro JDN (1987) Basal glucose homeostasis with and without fixed concentrations of glucagon and insulin. Metabolism 36:131–136
Katz J, McGarry JD (1984) The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest 74:1901–1909
Lickley HLA, Ross GG, Vranic M (1979) Effects of selective insulin and glucagon deficiency on glucose turnover. Am J Physiol 236:E255-E262
Liljenquist J, Müller G, Cherrington A, Perry JM, Rabinowitz D (1979) Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab 48:171–175
Müller MJ, Paschen U, Seitz HJ (1983) Glucose production measured by tracer and balance data in conscious miniature pigs. Am J Physiol 244:E236–244
Müller MJ, Paschen U, Seitz HJ (1984) Effect of ketone bodies on glucose production and utilization in the miniature pig. J Clin Invest 74:249–261
Müller MJ, Acheson KJ, Jequier E, Burger AG (1988a) Effect of thyroid hormones on oxidative and nonoxidative glucose metabolism in humans. Am J Physiol 255:E146–153
Müller MJ, Möhring J, Seitz HJ (1988b) Regulation of hepatic glucose output by glucose in vivo. Metabolism 37:55–60
Müller MJ, Mitchinson PE, Paschen U, Seitz HJ (1988c) Glucoregulatory function of glucagon in hypo-, eu- and hyperthyroid miniature pigs. Diabetologia 31:368–374
Polonsky K, Jaspan J, Pugh W, Dhoorajiwala J, Abraham M, Blix P, Moossa AR (1981) Insulin and glucagon breakthrough of somatostatin suppression. Importance of portal hormone measurements. Diabetes 30:664–669
Prager R, Wallace P, Olefsky JH (1986) In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest 78:472–481
Sacca L, Hendler R, Sherwin R (1978) Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab 47:1160–1163
Sherwin RS, Tamborlane W, Hendler R, Sacca L, DeFronzo RA, Felig P (1977a) Influence of glucagon replacement on the hyperglycemic and hyperketonemic response to prolonged somatostatin infusion in normal man. J Clin Endocrinol Metab 45:1104–1107
Sherwin RS, Hendler R, DeFronzo R, Wahren J, Felig P (1977b) Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci USA 74:348–352
Shulman GI, Liljenquist JE, Williams PE, Lacy WW, Cherrington AD (1978) Glucose disposal during insulinopenia in somatostatin treated dogs. The roles of glucose and glucagon. J Clin Invest 62:487–491
Soskin S, Essex H, Herrick J, Mann FC (1938) The mechanism of the regulation of blood sugar by the liver. Am J Physiol 214:558–567
Wolfe RR, Shaw JHF, Jahoor F, Herndon DN, Wolfe MH (1986) Response to glucose infusions in humans: role of changes in insulin concentration. Am J Physiol 250:E306–311
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Müller, M.J., Acheson, K.J., Burger, A.G. et al. Evidence that hyperglycaemia per se does not inhibit hepatic glucose production in man. Eur J Appl Physiol 60, 293–299 (1990). https://doi.org/10.1007/BF00379399
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00379399