Summary
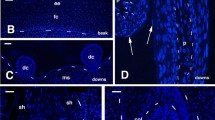
The distribution of glycosaminoglycans (GAGs) was studied in embryonic chick skin, using alcian blue staining with critical electrolyte concentration and glycanase treatment, immunofluorescence and transmission electron microscopy. Light microscopy revealed an uneven distribution of sulphated and non-sulphated GAGs at all stages of feather development. Along the dermal-epidermal junction and throughout the depth of the dermis, staining was stronger inside the feathers than in the interplumar skin. With increasing MgCl2 concentration, the decrease in stain intensity along the dermal-epidermal junction was stronger in interplumar skin than inside feather structures, indicating that sulphated GAGs are more abundant within feathers than in interplumar skin. The same differential sensitivity to electrolyte concentration was noted in the dermis, except at the feather placode stage, when labelling inside the dermal condensation was virtually wiped out at 0.6 M MgCl2 and higher concentrations, whereas it persisted in the surrounding dermis up to 0.8 M MgCl2, indicating that the dermal condensation contains a larger amount of hyaluronate than non-feather-forming dermis. Enzyme treatment of sections with Streptomyces hyaluronidase as compared with those treated with chondroitinase ABC corroborated these findings. Immunofluorescent detection of heparan sulphate proteoglycan revealed the presence of the antigen along the dermal-epidermal junction at all stages of feather development, with peaks of brightness in discrete spots of feather structures. Electron microscopy revealed the presence of ruthenium red and tannic acid positive material in the dermal-epidermal junctional zone and inside the dermis. The density of marked granules was somewhat higher in intraplumar than in interplumar regions. These observations demonstrate that certain sulphated and non-sulphated GAGs are distributed in a microheterogeneous manner, which appears to be related to the morphogenetic events of feather development. They are discussed in view of the possible role these components might play in dermal-epidermal interactions. They strengthen the notion, already gained from previous studies on the localization of interstitial collagens and fibronectin, that extracellular matrix components play an important structural and informative role in organogenesis.
Similar content being viewed by others
References
Ausprunk DH, Boudreau CL, Nelson DA (1981) Proteoglycans in the microvasculature. I. Histochemical localization in microvessels of the rabbit eye. Am J Pathol 103:353–366
Bashey RI, Fleischmajer R (1974) Increased synthesis of hyaluronic acid by insulin in embryonic chick skin. Proc Soc Exp Biol Med 145:18–20
Bernanke DH, Markwald RR (1979) Effects of hyaluronic acid on cardiac cushion tissue cells in collagen matrix cultures. Texas Rep Biol Med 39:271–281
Bernfield M, Banerjee SD, Koda JE, Rapraeger AC (1984) Remodeling of the basement membrane as a mechanism of morphogenetic tissue interaction. In: Trelstad RL (ed) Role of extracellular matrix in development. AR Liss, New York, pp 545–572
Brownell AG, Slavkin HC (1980) Role of basal lamina in tissue interactions. In: Lubec G (ed) Glomerular basement membrane, vol 3, n 1–6. Karger, Basel, pp 193–204
Cohn RH, Banerjee SD, Bernfield MR (1977) Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J Cell Biol 73:464–478
Conrad GW, Hamilton C, Haynes E (1977) Differences in glycosaminoglycans synthesized by fibroblast-like cells from chick cornea, heart and skin. J Biol Chem 252:6861–6870
Cook HC (1977) Carbohydrates. In: Bancroft ID, Stevens A (eds) Theory and practice of histological techniques. Churchill, London, pp 141–167
Davidson D (1984) Dermal cells form strong adhesions to the basement membrane during the development of feather primordia in chick skin. J Embryol Exp Morphol 84:149–158
Démarchez M, Mauger A, Sengel P (1981) The dermal-epidermal junction during the development of skin and cutaneous appendages in the chick embryo. Arch Anat Microsc Morphol Exp 70:205–218
Fisher M, Solursh M (1977) Glycosaminoglycan localization and role in maintenance of tissue spaces in the early chick embryo. J Embryol Exp Morphol 42:195–207
Gordon JR, Bernfield MR (1980) The basal lamina of the postnatal mammary epithelium contains glycosaminoglycans in a precise ultrastructural organization. Dev Biol 74:118–135
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hassell JR, Robey BG, Barrach HJ, Wilczek J, Rennard SI, Martin GR (1980) Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci (USA) 77:4494–4498
Hay ED (1978) Fine structure of embryonic matrices and their relation to cell surface in ruthenium red-fixed tissues. Growth 42:399–423
Kanwar YS, Farquhar MG (1979) Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the lamina rara by cationic probes. J Cell Biol 81:137–153
Kondo K, Seno N, Anno K (1971) Mucopolysaccharides from chicken skin of three age groups. Biochim Biophys Acta 244:513–522
Kosher RA, Savage MP, Walker RH (1981) A gradation of hyaluronate accumulation along the proximo-distal axis of the embryonic chick limb bud. J Embryol Exp Morphol 63:85–98
Lash JW, Saxén L, Ekblom P (1983) Biosynthesis of proteoglycans in organ cultures of developing kidney mesenchyme. Exp Cell Res 147:85–93
Lau EC, Ruch JV (1983) Glycosaminoglycans in embryonic mouse teeth and the dissociated dental constituents. Differentiation 23:234–242
Lau E, Arechaga J, Ruch JV (1983) Glycosaminoglycans in embryonic mouse tooth germs. A histochemical analysis. J Biol Buccale 11:23–34
Lemkin MC, Farquhar MG (1981) Sulfated and non sulfated glycosaminoglycans and glycopeptides are synthesized by kidney in vivo and incorporated into glomerular basement membranes. Proc Natl Acad Sci (USA) 79:1726–1730
Markwald RR, Fitzharris TP, Bolender DL, Bernanke DH (1979a) Structural analysis of cell: matrix association during the morphogenesis of atrioventricular cushion tissue. Dev Biol 69:634–654
Markwald RR, Funderberg FM, Bernanke DH (1979b) Glycosaminoglycans: potential determinants in cardiac morphogenesis. Texas Rep Biol Med 39:253–270
Markwald RR, Runyan RB, Kitten GT, Funderburg FM, Bernanke DH, Brauer PR (1984) Use of collagen gel cultures to study heart development. Proteoglycan and glycoprotein interactions during formation of endocardial cushion tissue. In: Trelstad RL (ed) Role of extracellular matrix in development. AR Liss, New York, pp 323–353
Mauger A, Démarchez M, Herbage D, Grimaud JA, Druguet M, Hartmann D, Sengel P (1982) Immunofluorescent localization of collagen types I and III and of fibronectin during feather morphogenesis in the chick embryo. Dev Biol 94:93–105
Mauger A, Démarchez M, Herbage D, Grimaud JA, Druguet M, Hartmann DJ, Foidart JM, Sengel P (1983) Immunofluorescent localization of collagen types I, III, IV, fibronectin and laminin during morphogenesis of scales and scaleless skin in the chick embryo. Wilhelm Roux's Arch 192:205–215
Mauger A, Démarchez M, Sengel P (1984) Role of extracellular matrix and of dermal-epidermal junction architecture in skin development. Progr Clin Biol Res 151:115–128
Meyer JM, Staubli A, Ruch JV (1981) Ruthenium red staining and tannic acid fixation of dental basement membrane. Cell Tissue Res 220:289–297
Nakamura A, Manasek FJ (1981) An experimental study of the relation of cardiac jelly to the shape of the early chick embryonic heart. J Embryol Exp Morphol 65:235–256
Nakamura T, Nagai Y (1980) Developmental changes in the synthesis of glycosaminoglycans and collagen in embryonic chick skin. J Biochem 87:629–637
Newman SA, Frisch HL, Perle MA, Tomasek JJ (1981) Limb development: Aspects of differentiation, pattern formation, and morphogenesis. In: Connelly TG, Brinkley LL, Carlson BM (eds) Morphogenesis and pattern formation. Raven Press, New York, pp 163–178
Olson GE, Low FN (1980) Relationships among extracellular components in the developing chick aorta. J Submicrosc Cytol 12:507–521
Ruch JV (1985) Odontoblast differentiation and the formation of the odontoblast layer. J Dental Res 64:489–498
Schoenwolf GC, Fisher M (1983) Analysis of the effects of Streptomyces hyaluronidase on formation of the neural tube. J Embryol Exp Morphol 73:1–15
Scott JE, Dorling J (1965) Differential staining of acid glycosaminoglycans (mucopolysaccharides) by Alcian blue in salt solutions. Histochemie 5:221–233
Sengel P (1976) Morphogenesis of skin. In: Abercrombie M, Newth DR, Torrey JG (eds) Developmental and cell biology series, Cambridge University Press, Cambridge, New York, London, Melbourne, 277 p.
Sengel P, Bescol-Liversac J, Guillam C (1962) Les mucopolysaccharides-sulfates au cours de la morphogenèse des germes plumaires chez l'embryon de poulet. Dev Biol 4:274–288
Silberstein GB, Daniel CW (1982) Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol 90:215–222
Silberstein GB, Daniel CW (1984) Glycosaminoglycans in the basal lamina and extracellular matrix of serially aged mouse mammary ducts. Mech Ageing Dev 24:151–162
Thesleff I, Barrach HJ, Foidart JM, Vaheri A, Pratt RM, Martin GR (1981) Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev Biol 81:182–192
Thompson HA, Spooner BS (1983) Proteoglycan and glycosaminoglycans synthesis in embryonic mouse salivary glands. Effect of β-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol 96:1443–1450
Toole BP (1981) Glycosaminoglycans in morphogenesis. In: Hay ED (ed) Cell biology of extracellular matrix. Academic Press, New York, pp 259–294
Toole BP (1982) Developmental role of hyaluronate. Connect Tissue Res 10:93–100
Toole BP, Goldberg RL, Chirosso G, Underbill CB, Orkin RW (1984) Hyaluronate-cell interactions. In: Trelstad RL (ed) Role of extracellular matrix in development. Alan R Liss, New York, pp 43–66
Trelstad RL, Hayashi K, Toole BP (1974) Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol 62:815–830
Turley EAI, Hollenberg MD, Pratt RM (1985) Effect of epidermal growth factor/urogastrone on glycosaminoglycans synthesis and accumulation in vitro in the developing mouse palate. Differentiation 28:279–285
Vaccaro CA, Brody JS (1979) Ultrastructural localization and characterization of proteoglycans in the pulmonary alveolus. Am Rev Resp Dis 120:901–910
Wasano K, Yamamoto T (1985) Microthread-like filaments connecting the epithelial basal lamina with underlying fibrillar components of the connective tissue in the rat trachea. A real anchoring device? Cell Tissue Res 239:485–495
Webster EH, Silver AF, Gonsalves NI (1983) Histochemical analysis of extracellular matrix material in embryonic mouse lens morphogenesis. Dev Biol 100:147–157
Wessells NK (1965) Morphology and proliferation during early feather development. Dev Biol 12:131–153
Wight TN, Ross R (1975) Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol 67:660–674
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jahoda, C.A.B., Mauger, A. & Sengel, P. Histochemical localization of skin glycosaminoglycans during feather development in the chick embryo. Roux's Arch Dev Biol 196, 303–315 (1987). https://doi.org/10.1007/BF00395954
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00395954