Summary
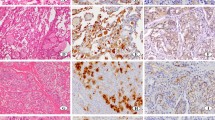
MAM-3 and MAM-6 antigens of human milk fat globule membrane were detected immunohistochemically in 93 cases of salivary gland tumours as well as in normal glands. The antigens were visualized in 10% formalin-fixed paraffin sections. MAM-3 (MoAbs 115G3, 67D11) antigen was distributed in intercalated and striated duct cells of the normal salivary glands, and in luminal tumour cells and squamous metaplastic cells of pleomorphic adenomas. In pleomorphic adenomas the frequency of positive staining with MoAb 67D11 (54/67; 80.6%) was higher than that with MoAb 115G3 (36/67; 53.7%). MAM-6 (MoAbs 115D8, 115F5) antigen was expressed in luminal and lateral borders of serous acinar cells and ductal of the normal glands, and also in luminal borders of tubulo-ductal and glandular structures of salivary gland tumours. Ductal basal cells were characterized by existence of positive staining for MAM-6 antigen, in adenolymphomas MAM-6 antigen was restricted to the basal tumour cells. Some mucous cells of mucoepidermoid tumours were stained specifically with MoAb 115G3, and epidermoid cells of mucoepidermoid carcinomas manifested MAM-6 antigen staining. Immunohistochemical localization of MAM-6 antigen resembled that of epithelial membrane antigen (EMA) detected with MoAb.
Similar content being viewed by others
References
Born IA, Schweheimer K, Maier H, Otto HF (1987) Cytokeratin expression in normal salivary glands and in cystadenolymphomas demonstrated by monoclonal antibodies against selective cytokeratin polypeptides. Virchows Arch [A] 411:583–589
Caselitz J, Walther B, Wustrow J, Seifert G, Weber K, Osborn M (1986) A monoclonal antibody that detects myoepithelial cells in exocrine glands, basal cells in other epithelia and basal and supra basal cells in certain hyperplastic tissues. Virchows Arch [A] 409:725–738
Caselitz J, Osborn M, Hamper K, Wustrow J, RauchfuΒ A, Weber K (1986) Pleomorphic adenomas, adenoid cystic carcinomas and adenolymphomas of salivary glands analysed by a monoclonal antibody against myoepithelial/basal cells. Virchows Arch [A] 409:805–816
Caselitz J, Osborn M, Wustrow J, Seifert G, Weber K (1986) Immunohistochemical investigations on the myoepithelial islands in lymphoepithelial lesions. Use of monoclonal keratin antibodies. Lab Invest 55:427–432
Dardick I, Rippstein P, Skimming L, Boivin M, Parks WR, Dairkee SH (1987) Immunohistochemical and ultrastructure of myoepithelium and modified myoepithelium of the ducts of human major salivary glands: Histologenetic implications for salivary gland tumors. Oral Surg 64:703–715
Dardick I, Parks WR, Little J, Brown DL (1988) Characterization of cytoskeletal proteins in basal cells of human parotid salivary gland duct. Virchow Arch [A] 412:525–532
Geiger S, Geiger B, Leitner O, Marshak G (1987) Cytokeratin polypeptides expression in different epithelial elements of human salivary glands. Virchow Arch [A] 410:403–414
Gusterson BA, Lucas RB, Ormerod MG (1982) Distribution of epithelial membrane antigen in benign and malignant lesions of the salivary glands. Virchow Arch [A] 397:227–233
Hilkens J, Buijs F, Hilgers J, Hageman Ph, Calafat J, Sonnenberg A, Van der Valk M (1984) Monoclonal antibodies against human milk fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer 34:197–206
Hilkens J, Hilgers J, Buijs F, Hageman Ph, Schol D, van Doornewaard G, van den Tweel J (1984) Monoclonal antibodies against human milk fat globule membranes useful in carcinoma reaction. In: Peerers H (ed) Protides of the Biological Fluids, vol. 31. Pergamon Press, Oxford/New York, pp 728–735
Knight J, Gusterson B, Jones RR, Landells W, Wilson P (1985) Monoclonal antibodies specific for subsets of epidermal keratins: Biochemical and immunocytochemical characterization. Applications in pathology and cell culture. J Pathol 145:341–354
Leoncini P, Cintorino M, Vindigni C, Leoncini L, Armellini D, Bugnoli M, Skalli O, Gabbiani G (1988) Distribution of cytoskeletal and contractile proteins in normal and tumour bearing salivary and lacrimal glands. Virchows Arch [A] 412:329–337
Marshak G, Leitner O (1987) Cytokeratin polypeptides in normal and metaplastic human salivary gland epithelium. J Oral Pathol 16:442–449
Marshall RJ, Herbert A, Braye SG, Jones DB (1984) Use of antibodies to carcinoembryonic antigen and human milk fat globule to distinguish carcinoma, mesohelioma, and reactive mesothelium. J Clin Pathol 37:1215–1221
Orito T, Shinohara H, Okada Y, Mori M (1989) Heterogeneity of keratin expression in epithelial tumor cells of adenolymphoma in paraffin sections. Pathol Res Pract 84:600–608
Palmer RM (1986) The identification of myoepithelial cells in human salivary glands. A review and comparison of light microscopical methods. J Oral Pathol 15:221–229
Palmer RM, Lucas R, Knight J, Gusterson B (1985) Immunocytochemical identification of cell types in pleomorphic adenoma, with particular reference to myoepithelial cells. J Pathol 146:213–220
Pinkus GS, Kurtin PJ (1985) Epithelial membrane antigen — a diagnostic discriminant in surgical pathology. Immunohistochemical profile in epithelial, mesenchymal and hetmatopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol 16:929–940
Sloane JP, Ormerod MG (1981) Distribution of epithelial membrane antigen in normal and neoplastic tissues and its value in diagnostic tumor pathology. Cancer 47:1786–1795
Sloane JP, Ormerod MG, Carter RL, Gusterson BA, Foster CS (1982) An immunocytochemical study of the distribution of epithelial membrane antigen in normal and disordered squamous epithelium. Diag Histopathol 5:11–17
Sumitomo S, Kumasa S, Mitani H, Mori M (1987) Comparison of CEA distribution in lesions and tumors of salivary glands as determined with monoclonal and polyclonal antibodies. Virchow Arch [B] 53:133–139
Takai T, Yamada Y, Shinohara Y, Orito T, Tsukitani K, Mori M (1988) Monoclonal antibodies against keratins bind to intercalated duct and ductal basal cells of normal salivary glands in paraffin sections. Acta Histochem Cytochem 21:573–584
Tatemoto Y, Kumasa S, Watanabe Y, Mori M (1987) Immunohistochemical expression of monoclonal antibody against epithelial membrane antigen in salivary gland tumors. Acta Histochem Cytochem 20:113–124
Tatemoto Y, Kumasa S, Watanabe Y, Mori M (1987) Epithelial membrane antigen as a marker of human salivary gland acinar and ductal cell function. Acta Histochem 82:219–226
Taylor-Papadimitriou J, Peterson JA, Arklie J, Burchell J, Ceriani RL, Bormer WF (1981) Monoclonal antibodies to epithelium-specific component of human milk fat globule membrane: Production and reaction with cells in culture. Int J Cancer 28:17–21
Tron V, Wright JL, Churg A (1987) Carcinoembryonic antigen and milk-fat globule protein staining of malignant mesothelioma and adenocarcinoma of the lung. Arch Pathol Lab Med 111:291–293
Tsubura A, Morii S, Hilkens J, Hilgers J (1985) Expression of MAM-3 and MAM-6 antigens on endometrial and endocervical carcinomas. Virchow Arch [A] 407:59–67
Tsubura A, Morii S, Ueda S, Sasaki M, Zotter St, Watzig V, Mooi W, Hageman PhC, Hilkens J, Van der Tweel J, Meijer C, Hilgers J (1987) lmmunohistochemical demonstration of MAM-3 and MAM-6 antigens in normal human skin appendages and their tumors. Arch Dermatol Res 279:550–557
Tsuji T, Shinozaki F, Yamada Y, Mori M (1989) Immunohistochemical detection of human lung and gastric cancer antigen in human salivary gland tumors. Anticancer Res (in press)
Tsukitani K, Kobayashi K, Murase N, Sumitomo S, Mitani H, Mori M (1985) Characterization of cell in salivary gland lesions by immunohistochemical identification of carcinoembryonic antigens. Oral Surg 59:595–599
Zotter S, Lossnitzer A, Kunze KS, Müller M, Hilkens J, Hilgers J, Hageman P (1985) Epithelial markers for paraffin-embedded human tissues. Immunohistochemistry with monoclonal antibodies against milk fat globule antigens. Virchow Arch [A] 406:237–251
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamada, K., Tanaka, T., Mori, M. et al. Immunohistochemical expression of MAM-3 and MAM-6 antigens in salivary gland tumours. Vichows Archiv A Pathol Anat 415, 509–521 (1989). https://doi.org/10.1007/BF00718644
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00718644