Abstract
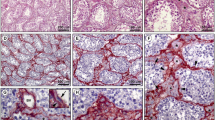
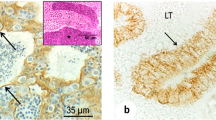
Testicular peritubular cells are located in the lamina propria of seminiferous tubules. These cells, significantly contributing to the basal membrane of seminiferous epithelium, have been studied in a number of species. However, there is a lack of data on the development of the lamina propria in the human testis. The aim of our survey was to investigate the characteristics of the lamina propria and, in particular, peritubular cells in the fetal human testes by immunohistological and stereological methods. Therefore, testes (14–39 weeks of gestation, n=45) were dissected and fixed in a 4% buffered paraformaldehyde solution. Several pieces of each testis were embedded in paraffin and processed for immunohistochemical and stereological analysis. All investigated testes have shown sex cords in the process of development and differentiation. Morphologically, peritubular cells in the lamina propria can be divided into two types: fibroblast-like (FL) and myoid-like (ML) type (cells which much resemble mature myoid cells). By immunohistochemistry, both FL and ML cells are found to be strongly positive for the intermediate filament desmin, but negative for α-smooth actin. While FL cells intensively express Ki-67 demonstrating proliferative activity, ML cells are found to be negative. The basement membrane of sex cords as well as the blood vessels of the interstitium show strong positivity to collagen IV and laminin. Concerning the correlation between the appearance of the investigated antigens with the gestational age, all antigens have been expressed (in the manner described above) already in the 14th week of gestation. The stereological analysis of the number (Nv) and volume (Vv) of peritubular cells indicates a pulsatile development of these cells in the lamina propria of the human fetal testis. While the stereological variables determined for FL cells show a gradual decrease, the same variables determined for ML cells demonstrate a successive increase. It appears that the lamina propria of the fetal human testes shares many of the properties previously discovered in rodents.
Similar content being viewed by others
References
Abercrombie M (1946) Estimation of nuclear population from microtomic sections. Anat Rec 94: 239–247
Bardin CW (1986) Pituitary-testicular axis. In: Yen SSC, Jaffe RB (eds) Reproductive endocrinology. Saunders, Philadelphia, pp 177–199
Bellvé AH, Feig LA (1984) Cell proliferation in the mammalian testis: biology of the seminiferous growth factor (SGF). Recent Prog Horm Res 40: 531–567
Bogan JS, Page DC (1994) Ovary? Testis? — a mammalian dilemma. Cell 76: 603–607
Böck P, Breitenecker G, Lunglmayr G (1972) Kontraktile Fibroblasten (Myofibroblasten) in der Lamina propria der Hodenkanälchen vom Menschen. Z Zellforsch Mikrosk Anat 133: 519–527
Bustos-Obregón E, Holstein AF (1973) On structural patterns of the lamina propria of human seminiferous tubules. Z Zellforsch Mikrosk Anat 141: 413–425
Cailleau J, Vermeire S, Verhoeven G (1990) Independent control of the production of insulin-like growth factor I and its binding protein by cultured testicular cells. Mol Cell Endocrinol 69: 79–85
Clermont Y (1958) Contractile elements in the limiting membrane of the seminiferous tubule of the rat. Exp Cell Res 15: 438–440
Clermont Y, Perry B (1957) Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat 100: 241–247
Davidoff MS, Breucker H, Holstein AF, Seidel K (1990) Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res 262: 253–261
Davidoff MS, Schulze W, Middendorff R, Holstein AF (1993) The Leydig cell of the human testis — a new member of the diffuse neuroendocrine system. Cell Tissue Res 271: 429–439
DeHoff RT, Rhines FS (1968) Quantitative microscopy. McGraw Hill, New York
Elias H, Hyde DM (1980) Elementary stereology. Am J Anat 159: 411–446
Fawcett DW, Burgos MH (1960) Studies on the fine structure of the mammalian testis. II. The human interstitial tissue. Am J Anat 107: 245–254
Fawcett DW, Heidger PM, Leak LV (1969) Lymph vascular system of the interstitial tissue of the testis as revealed by electron microscopy. J Reprod Fertil 19: 109–119
Franke MW, Schmid E, Winter S, Osborn M, Weber K (1979) Widespread occurrence of intermediate-sized filaments of the vimentin type in cultured cells from diverse vertebrates. Exp Cell Res 123: 25–46
Gerdes J, Schwab U, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31: 13–20
Gier HT, Marion GB (1970) Development of mammalian testis. In: Johnson AD, Gomes WR, Vandemark NL (eds) The testis. Academic Press, New York, pp 113–129
Hadžiselimović F (1977) Cryptorchidism. Springer, Berlin Heidelberg New York
Hettle JA, Balekjian E, Tung PS, Fritz IB (1988) Rat testicular peritubular cells in culture secrete an inhibitor of plasminogen activator activity. Biol Reprod 38: 359–364
Hittmair A, Schmid KW (1989) Inhibition of endogenous peroxidase for the immunohistochemical demonstration of intermediate filament proteins (IFP). J Immunol Methods 116: 199–205
Longtine JA, Pincus GS, Fujiwara K, Corson JM (1985) Immunohistochemical localization of smooth muscle myosin in normal human tissues. J Histochem Cytochem 33: 179–184
Niemi M, Kormano M (1965) Contractility of the seminiferous of the postnatal testis and its response to oxytocin. Ann Med Exp Biol Finniae (Helsinki) 43: 40–42
Norton JN, Skinner MK (1989) Regulation of Sertoli cell function and differentiation through the actions of a testicular paracrine factor PModS. Endocrinology 124: 2711–2719
Posinovec J, Banek Lj, Kablar B (1991) Development of the peritubular myoid cells in the rat testis. Jugosl Ginekol Perinatol 31: 18–22
Roosen-Runge EC (1951) Motions of the seminiferous tubules of rat and dog. Anat Rec 109: 413
Ross MH (1967) The fine structure and development of the peritubular contractile cell component in the seminiferous tubules in the mouse Am J Anat 121: 523–533
Ross MH, Long JR (1966) Study on normal and irradiated boundary tissue of the seminiferous tubules of the rat. Science 153: 1271–1273
Sachs L (1989) Statistische Methoden: Planung und Auswertung. Springer, Berlin Heidelberg New York
Schulze W, Davidoff MS, Holstein A-F (1987) Are Leydig cells of neural origin? Substance P-like immunoreactivity in human testicular tissue. Acta Endocrinol (Copenh) 115: 373–377
Seidel K, Holstein AF (1990) Evidence for the presence of nerve growth factor (NGF) and NGF receptor in human testis. Cell Tissue Res 261: 539–547
Shi SR, Key ME, Kalra KL (1991) Antigen-retrieval in formalinfixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39: 741–748
Skinner MK, Fritz IB (1985a) Structural characterization of proteoglycans produced by testicular peritubular cells and Sertoli cells. J Biol Chem 260: 1874–1883
Skinner MK, Fritz IB (1985b) Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell function. Proc Natl Acad Sci USA 82: 114–118
Skinner MK, Moses HL (1989) Tranforming growth factor-beta gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Mol Endocrinol 3: 625634
Skinner MK, Tung PS, Fritz IB (1985) Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100: 1941–1947
Skinner MK, Fetterolf PM, Anthony CT (1988) Purification of a paracrine factor, PModS, produced by testicular peritubular cells, that modulates Sertoli cell function. J Biol Chem 263: 2884–2889
Skinner MK, Takacs K, Coffey RJ (1989) Cellular localization of transforming growth factor-alpha gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Endocrinology 124: 845–852
Steger K, Wrobel KH (1994) Immunohistochemical demonstration of cytoskeletal proteins in the ovine testis during postnatal development. Anat Embryol 189: 521–530
Steger K, Schimmel M, Wrobel KH (1994) Immunocytochemical demonstration of cytoskeletal proteins in seminiferous tubules of adult rams and bulls. Arch Histol Cytol 57: 17–28
Toyama Y (1977) Actin-like filaments in the myoid cell of the testis. Cell Tissue Res 177: 227–238
Tung PS, Fritz IB (1980) Interactions of Sertoli cells with myoid cells in vitro. Biol Reprod 23: 207–217
Tung PS, Fritz IB (1987) Morphogenetic restructuring and formation of basement membranes by Sertoli cells and testis peritubular cells in co-culture: inhibition of the morphogenetic cascade by cyclic AMP derivatives and by blocking direct cell contact. Dev Biol 120: 139–153
Tung PS, Skinner MK, Fritz IB (1984) Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann NY Acad Sci 438: 435–446
Vergouwen RPEA, Huiskamp R, Bas RJ, Roepersgajadien HL, Davids JAG, Derooij DG (1993) Postnatal development of testicular cell populations in mice. J Reprod Fertil 99: 479–485
Virtanen I, Kallajoki M, Närvänen O, Paranko J Thornell LE, Miettinen M, Lehto VP (1986) Peritubular myoid cells of human rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec 215: 10–20
Vuolteenaho R, Nissien M, Sainio K, Byers M, Eddy R, Hirvonen H, Shows TB, Sariola H, Engvall E, Tryggvason K (1994) Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol 124: 381–394
Weibel ER (1979) Stereological methods. Practical methods for biological morphometry, vol 1. Academic Press, London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jezek, D., Hittmair, A., Rogatsch, H. et al. Lamina propria of sex cords in human fetal testis: an immunohistological and stereological study. Anat Embryol 193, 181–190 (1996). https://doi.org/10.1007/BF00214709
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214709