Abstract
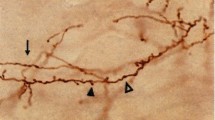
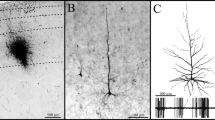
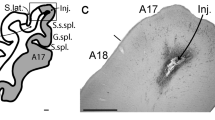
The trajectory of long distance intrahemispheric corticocortical axons has been investigated using the anterograde fluorescent axonal tracer fluororuby. Most axons of this kind were found to travel through the gray matter of layers VI and VII rather than in the white matter. The cell-sparse zone immediately superficial to layer VII contains a dense aggregate of longitudinally directed axons. Corticocortical axons traveling in the mediolateral plane also utilize the deep gray matter predominately. Layer VII neurons are persistent remnants of the subplate in rats. Based on our retrograde labeling results, they are involved in long distance as well as local corticocortical connections. Layer VII neurons are often labeled in a more continuous pattern after cortical injections of retrograde tracers than neurons of layers II, III and V, which are labeled in a patchy manner.
Similar content being viewed by others
References
Akers RM, Killackey HP (1978) Organization of corticocortical connections in the parietal cortex of the rat. J Comp Neurol 181: 513–538
Allendoerfer KL, Shatz CJ (1994) The subplate, a transient neo-cortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 17: 185–218
Berendse HW, Groenewegen HJ (1991) Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42: 73–102
Burton H (1985) Second somatosensory cortex and related areas. In: Peters A, Jones EG (eds) Cerebral cortex, vol 5. Sensory-motor areas and aspects of cortical connectivity. Plenum Press, New York, pp 31–98
Cajal S, Ramón Y (1892) El nuevo concepto de la histologia de los centros nerviosos. Rev Ciencias Med 18: 457–476
Cavada C, Goldman-Rakic PS (1989) Posterior parietal cortex in rhesus monkey. II. Evidence for segregated corticortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445
DeFilipe J, Jones EG (1988) Cajal on the cerebral cortex. Oxford University Press, Oxford, New York
DeFilipe J, Conley M, Jones EG (1986) Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J Neurosci 6: 3749–3766
Divac I, Marinkovic S, Mogensen J, Schwerdtfeger W, Regidor J (1987) Vertical ascending connections in the isocortex. Anat Embryol 175: 443–455
Goldman PS, Nauta WJH (1977) Columnar distribution of corticocortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res 122: 393–413
Herkenham, M (1979) The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol 183: 487–518
Hess DT, Merker BH (1983) Technical modifications of Gallyas' silver stain for myelin. J Neurosci Methods 8: 95–97
Jacobson S (1965) Intralaminar, interlaminar, callosal, and thalamocortical connections in frontal and parietal areas of the albino rat cerebral cortex. J Comp Neurol 124: 131–146
Jones EG (1986) Connectivity of the primate sensory-motor cortex. In: Peters A, Jones EG (eds) Cerebral cortex, vol 5. Sensory-motor areas and aspects of cortical connectivity. Plenum Press, New York, pp 113–183
Jones EG, Coulter JD, Hendry SHC (1978) Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181: 291–348
Kostovic I, Rakic P (1990) Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297: 441–470
Krieg WJS (1946) Connections of the cerebral cortex. I. The albino rat. B. Structure of the cortical areas. J Comp Neurol 84: 277–323
Krieg WJS (1963) Connections of the cerebral cortex. Wendell Krieg, Evanston, Illinois
Leichnetz GR, Astruc J (1975) Efferent connections of the orbitofrontal cortex in the marmoset monkey (Saguinus oedipus). Brain Res 84: 169–180
Lund JS, Fitzpatrick D, Humphrey AL (1985) The striate visual cortex of the tree shrew. In: Peters A, Jones EG (eds) Cerebral cortex, vol 3. Visual cortex. Plenum Press, New York, pp 157–205
Marin-Padilla M (1978) Dual origin of the mammalian cerebral neocortex and evolution of the cortical plate. Anat Embryol 152: 109–126
Mascagni F, McDonald AJ, Coleman JR (1993) Corticoamygdaloid and corticocortical projections of rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience 57: 697–715
McGeorge AJ, Faull RLM (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29: 503–537
Miller MW, Vogt BA (1984) Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol 226: 184–202
Pandya DN, Yeterian EH (1985) Architecture and connections of cortical association areas. In: Peters A, Jones EG (eds) Cerebral cortex, vol 4. Association and auditory cortices. Plenum Press, New York, pp 3–61
Pearlman AL (1985) The visual cortex of the normal mouse and the reeler mutant. In: Peters A, Jones EG (eds) Cerebral cortex, vol 3. Visual cortex. Plenum Press, New York, pp 1–18
Peters A (1985) The visual cortex of the rat. In: Peters A, Jones EG (eds) Cerebral cortex, vol 3. Visual cortex. Plenum Press, New York, pp 19–80
Reep RL, Goodwin GS (1988) Layer VII of rodent cerebral cortex. Neurosci Lett 90: 15–20
Reep RL, Corwin JV, Hashimoto A, Watson RT (1987) Efferent connections of the rostral component of medial agranular cortex in rats. Brain Res Bull 19: 203–221
Reep RL, Goodwin GS, Corwin JV (1990) Topographic organization in the corticocortical connections of medial agranular cortex in rats. J Comp Neurol 294: 262–280
Reep RL, Chandler HC, King V, Corwin JV (1994) Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res 100: 67–84
Rockland KS (1992) Configuration, in serial reconstruction, of individual axons projecting from area V2 to V4 in the macaque monkey. Cereb Cortex 2: 353–374
Rockland KS (1995) Morphology of individual axons projecting from area V2 to MT in the macaque. J Comp Neurol 355: 15–26
Romanski LM, LeDoux JE (1993) Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex 3: 515–532
Rose M (1928) Die Inselrinde des Menschen und der Tiere. J Psychol Neurol 37: 467–624
Rose M (1929) Cytoarchitektonischer Atlas der Grosshirnrinde der Maus. J Psychol Neurol 40: 1–51
Rosenquist AC (1985) Connections of visual cortical areas in the cat. In: Peters A, Jones EG (eds) Cerebral cortex, vol 3. Visual cortex. Plenum Press, New York, pp 81–117
Schmued LC (1990) A rapid, sensitive histochemical stain for myelin in frozen brain sections. J Histochem Cytochem 38: 717–720
Schwark HD, Jones EG (1989) The distribution of intrinsic cortical axons in area 3b of cat primary somatosensory cortex. Exp Brain Res 78: 501–513
Selemon LD, Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8: 4049–4068
Sukekawa K (1988) Interconnections of the visual cortex with the frontal cortex in the rat. J Hirnforsch 29: 83–93
Torrealba F, Olavarria J, Carrasco MA (1984) Cortical connections of the anteromedial extrastriate visual cortex in the rat. Exp Brain Res 56: 543–549
Valverde F, Facal-Valverde MV (1988) Postnatal development of interstitial (subplate) cells in the white matter of the temporal cortex of kittens: a correlated Golgi and electron microscopic study. J Comp Neurol 269: 168–192
Valverde F, Facal-Valverde M, Santacana M, Heredia M (1989) Development and differentiation of early generated cells of sublayer VIb in the somatosensory cortex of the rat: a correlated golgi and autoradiographic study. J Comp Neurol 290: 118–140
Valverde F, López-Mascaraque L, Santacana M, De Carlos JA (1995) Persistence of early-generated neurons in the rodent subplate: assessment of cell death in neocortex during the early postnatal period. J Neurosci 15: 5014–5024
Van Eden CG, Lamme VAF, Uylings HBM (1992) Heterotopic cortical afferents to the medial prefrontal cortex in the rat. A combined retograde and anterograde tracer study. Eur J Neurosci 4: 77–97
Van Essen DC (1985) Functional organization of primate visual cortex. In: Peters A, Jones EG (eds) Cerebral cortex, vol 3. Visual cortex. Plenum Press, New York, pp 259–329
Van Hoesen GW, Pandya DN, Butters N (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res 95: 25–38
Vaz Ferreira A (1951) The cortical areas of the albino rat studied by silver impregnation. J Comp Neurol 95: 177–243
Vogt BA (1985) Cingulate cortex. In: Peters A, Jones EG (eds) Cerebral cortex, vol 4. Association and auditory cortices. Plenum Press, New York, pp 89–149
Vogt BA, Miller MW (1983) Cortical connections between rat cin-gulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216: 192–210
Wagner GP, Eins S, Wolff JR (1986) Tangential organization of the infragranular fiber plexus in rat cerebral cortex. Acta Anat 126: 1–12
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vandevelde, I.L., Duckworth, E. & Reep, R.L. Layer VII and the gray matter trajectories of corticocortical axons in rats. Anat Embryol 194, 581–593 (1996). https://doi.org/10.1007/BF00187471
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00187471