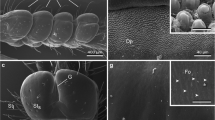
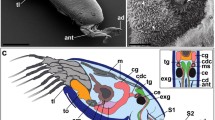
Summary
Coronal podia of Sphaerechinus granularis are anchoring (adhering) appendages involved in either locomotion or capture of drift materials. Adhesion is not due to the presumed sucker action of the disc but relies entirely on secretions of the disc epidermis. Peristomeal podia function in wrapping together food particles or food fragments in an adhesive material thus facilitating their capture by the Aristotle's lantern. In both types of podia, the disc epidermis is made up of four cell types: non-ciliated secretory cells (NCS cells) that contain graules whose content is at least partly mucopolysaccharidic in nature, ciliated secretory cells (CS cells) containing granules of unknown nature, ciliated non-secretory cells (CNS cells) and support cells. The cilia of CS cells are subeuticular whereas those of CNS cells, although also short and rigid, traverse the cuticle and protrude in the outer medium. All these cells are presumably involved in an adhesive/de-adhesive process functioning as a duogland adhesive system. Adhesive secretion would be produced by NCS cells and de-adhesive secretion by CS cells. These secretions would be controlled through stimulations by the two types of ciliated cells (receptor cells) which presumably interact with the secretory cells by way of the nerve plexus. This model of adhesion/de-adhesion fits well with the activities of both coronal and peristomeal podia. The secretion of NCS cells would make up a bridge of adhesive material between a podium and the substratum (coronal podia) or would coat and gather food particles (peristomeal podia), respectively. The de-adhesive material enclosed in the granules of CS cells would allow the podia (either coronal or peristomeal) to easily become detached from the substratum and to always remain clear of any particles.
Similar content being viewed by others
References
Ball B, Jangoux M (1990) Ultrastructure of the tube foot sensory-secretory complex in Ophiocomina nigra (Echinodermata, Ophiuroidea). Zoomorphology 109:201–209
Buchanan JB (1969) Feeding and the control of volume within the tests of regular sea-urchins. J Zool (London) 159:51–64
Burke RD (1980) Podial sensory receptors and the induction of metamorphosis in echinoids. J Exp Mar Biol Ecol 47:223–234
Cameron JL, Fankboner PB (1984) Tentacle structure and feeding processes in life stages of the commercial sea cucumber Parastichopus californicus (Stimpson). J Exp Mar Biol Ecol 81:193–209
Campbell SS, Crawford BJ (1991) Ultrastructural study of the hyaline layer of the starfish embryo, Pisaster ochraceus. Anat Rec 231:125–135
Coleman R (1969) Ultrastructure of the tube foot sucker of a regular echinoid, Diadema antillarum Philippi, with special reference to the secretory cells. Z Zellforsch Mikrosk Anat 96:151–161
Dambach M, Hentschel G (1970) Die Bedeckungsreaktion von Seeigeln. Neue Versuche und Deutungen. Mar Biol 6:135–141
De Ridder C, Lawrence JM (1982) Food and feeding mechanisms: Echinoidea. In: Jangoux M, Lawrence JM (eds) Echinoderm Nutrition. Balkema, Rotterdam, pp 57–115
De Ridder C, Jangoux M, De Vos L (1987) Frontal ambulacral and peribuccal areas of the spatangoid echinoid Echinocardium cordatum (Echinodermata): A functional entity in feeding mechanism. Mar Biol 94:613–624
Dietrich HF, Fontaine AR (1975) A decalcification method for ultrastructure of echinoderm tissues. Stain Technol 50:351–354
Engster MS, Brown SC (1972) Histology and ultrastructure of the tube foot epithelium in the phanerozonian starfish, Astropecten. Tissue Cell 4:503–518
Fenner DH (1973) The respiratory adaptations of the podia and ampullae of echinoids (Echinodermata). Biol Bull 145:323–339
Flammang P, Jangoux M (1992) Functional morphology of the locomotory podia of Holothuria forskali (Echinodermata, Holothuroida). Zoomorphology 111:167–178
Flammang P, De Ridder C, Jangoux M (1991) Ultrastructure of the penicillate podia of the spatangoid echinoid Echinocardium cordatum (Echinodermata) with special emphasis on the epidermal sensory-secretory complexes. Acta Zool (Stockholm) 72:151–158
Ganter P, Jollès G (1969–1970) Histochimie normale et pathologique, vols 1 et 2. Gauthiers-Villars, Paris
Gelder SR, Tyler S (1986) Anatomical and cytochemical studies on the adhesive organs of the ectosymbiont Histriobdella homari (Annelida: Polychaeta). Trans Am Microsc Soc 105:348–356
Hermans CO (1983) The duo-gland adhesive system. Oceanogr Mar Biol Ann Rev 21:281–339
Holland ND, Nealson KH (1978) The fine structure of the echinoderm cuticle and the subcuticular bacteria of echinoderms. Acta Zool (Stockholm) 59:169–185
Lawrence JM (1987) A functional biology of echinoderms. Croom Helm. London
McKenzie JD (1987) The ultrastructure of the tentacles of eleven species of dendrochirote holothurians studied with special reference to surface coats and papillae. Cell Tissue Res 248:187–199
McKenzie JD (1988) The ultrastructure of tube foot epidermal cells and secretions: Their relationship to the duo-glandular hypothesis and the phylogeny of the echinoderm classes. In: Paul CRC, Smith AB (eds) Echinoderm phylogeny and evolutionary biology. Clarendon Press, Oxford, pp 287–298
Mooi R (1986) Non-respiratory podia of clypeasteroids (Echinodermata, Echinoides): II. Diversity. Zoomorphology 106:75–90
Nichols D (1961) A comparative histological study of the tube feet of two regular echinoids. Quart J Microsc Sci 102:157–180
Nichols D (1966) Functional morphology of the water-vascular system. In: Boolootian RA (ed) Physiology of Echinodermata. Interscience Publishers, New York, pp 219–240
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie Brilliant blue G250. Anal Biochem 79:544–552
Smith AB (1979) Peristomial tube feet and plates of regular echinoids. Zoomorphologie 94:67–80
Thomas LA, Hermans CO (1985) Adhesive interactions between the tube feet of a starfish, Leptasterias hexactis, and substrata. Biol Bull 169:675–688
Tyler S (1976) Comparative ultrastructure of adhesive systems in the Turbellaria. Zoomorphologie 84:1–76
Tyler S (1988) The role of function in determination of homology and convergence — examples from invertebrates adhesive organs. Fortschr Zool 36:331–347
Tyler S, Rieger GE (1980) Adhesive organs of the Gastrotricha. I. Duo-gland organs. Zoomorphologie 95:1–15
Author information
Authors and Affiliations
Additional information
Research Assistant, National Fund for Scientific Research (Belgium)
Rights and permissions
About this article
Cite this article
Flammang, P., Jangoux, M. Functional morphology of coronal and peristomeal podia in Sphaerechinus granularis (Echinodermata, Echinoida). Zoomorphology 113, 47–60 (1993). https://doi.org/10.1007/BF00430976
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00430976