Abstract
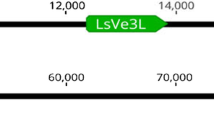
The fungal disease resistance locus Alternaria stem canker (Asc) in tomato has been suggested to encode the enzyme aspartate carbamoyltransferase (AC Tase). To test this hypothesis a segment of the tomato ACTase gene was amplified by the polymerase chain reaction (PCR) using degenerate primers. The PCR product obtained was subsequently used to isolate an ACTase cDNA clone. Restriction fragment length polymorphism (RFLP) linkage analysis showed that the ACTase gene and the Asc locus do not cosegregate. RFLP mapping positioned the ACTase gene on chromosome 11, while the Asc locus is located on chromosome 3. These results exclude the possibility that the ACTase protein is encoded by the Asc locus.
Similar content being viewed by others
References
Aarts JMMJG, Hontelez JGJ, Fischer P, Verkerk R, Kammen A van, Zabel P (1991) Acid phosphatase-11, a tightly linked molecular marker for root-knot nematode resistance in tomato: from protein to gene, using PCR and degenerate primers containing deoxyinosine. Plant Mol Biol 16:647–661
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Clouse SD, Gilchrist DG (1987) Interaction of the asc locus in F8 paired lines of tomato with Alternaria alternata f. sp. lycopersici and AAL-toxin. Phytopathology 77:80–82
Collins KD, Stark GR (1971) Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-Laspartate. J Biol Chem 246:6599–6605
Daly JM (1984) The role of recognition in plant disease. Annu Rev Phytopathol 22:273–307
Davis LG, Dibner MD, Battey JF (1986) Basic Methods in Molecular Biology. Elsevier, New York
Dellaporta SL, Woods J, Hicks JB (1983) A plant DNA minipreparation version II. Plant Mot Biol Rep 1(4):19–21
Faure M, Camonis JH, Jacquet M (1989) Molecular characterization of a Dictyostelium discoideum gene encoding a multifunctional enzyme of the pyrimidine pathway. Eur J Biochem 179:345–358
Ferguson AR, Johnston JS (1980) Phaseolotoxin: Chlorosis, ornithine accumulation and inhibition of ornithine carbamoyltransferase in different plants. Physiol Plant Pathol 16:269–275
Freund JN, Jarry BP (1987) The rudimentary gene of Drosophila melanogaster encodes four enzymic functions. J Mol Biol 193:1–13
Fuson GB, Pratt D (1988) Effects of the host-selective toxins of Alternaria alternata f. sp. lycopersici on suspension-cultured tomato cells. Phytopathology 78:1641–1648
Gilchrist DG (1983) Molecular modes of action. In: Daly JM, Deverall BJ (eds) Toxins and plant pathogenesis. Academic Press, Sydney, pp 81–136
Gilchrist DG, Grogan RG (1976) Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology 66:165–171
Grogan RG, Kimble KA, Misaghi I (1975) A stem canker disease caused by Alternaria alternata f. sp. lycopersici. Phytopathology 65:880–886
Haring MA, Rommens CMT, Nijkamp HJJ, Hille J (1991) The use of transgenic plants to understand transposition mechanisms and to develop transposon tagging strategies. Plant Mol Biol 16:449–461
Johal GS, Briggs SP (1992) Reductase activity encoded by the HM1 disease resistance gene in maize. Science 258:985–987
Kater MM, Koningstein GM, Nijkamp HJJ, Stuitje AR (1991) cDNA cloning and expression of Brassica napus enoyl-acyl carrier protein reductase in Escherichia coli. Plant Mol Biol 17:895–909
Keen NT (1992) The molecular biology of disease reistance. Plant Mol Biol 19:109–122
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
McFarland B (1984) Studies on the interaction of tomato and Alternaria alternata f. sp. lycopersici host-selective toxins. PhD dissertation, Dept of Plant Pathology, University of California, Davis, California, USA
Moussatos VV (1989) AAL-toxin-associated cell death processes in Lycopersicon esculentum Mill. PhD dissertation, Dept. of Plant Pathology, University of California, Davis, California, USA
Nagy M, Le Gouar M, Potier S, Souciet J-L, Hervé G (1989) The primary structure of the aspartate transcarbamylase region of the URA2 gene product in Saccharomyces cerevisiae. J Biol Chem 264:8366–8374
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Simmer JP, Kelly RE, Scully JL, Grayson DR, Rinker AG Jr, Bergh ST, Evans DR (1989) Mammalian aspartate transcarbamylase (ACTase): Sequence of the ACTase domain and interdomain linker in the CAD multifunctional polypeptide and properties of the isolated domain. Proc Natl Acad Sci USA 86:4382–4386
Tanksley SD, Ganal MW, Prince JP, De Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovanonni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Miller L, Paterson AH, Pineda O, Roder MS, Wing RA, Wu W, Young ND (1992) High density maps of the tomato and potato genomes. Genetics 132:1141–1160
Turner JG (1981) Tabtoxin, produced by Pseudomonas tabaci, decreases Nicotiana tabacum glutamine synthetase in vivo and causes accumulation of ammonia. Physiol Plant Pathol 19:57–67
Turner JG (1986) Activities of ribulose-1,5-bisphosphate carboxylase and glutamine synthetase in isolated mesophyll cells exposed to tabtoxin. Physiol Mol Plant Pathol 29:59–68
Witsenboer HMA, Griend EG van de, Tiersma JB, Nijkamp HJJ, Hille J (1989) Tomato resistance to Alternaria stem canker: Localization in host genotypes and functional expression compared to non-host-resistance. Theor Appl Genet 78:457–462
Author information
Authors and Affiliations
Additional information
Communicated by H Saedler
Rights and permissions
About this article
Cite this article
Overduin, B., Hogenhout, S.A., van der Biezen, E.A. et al. The Asc locus for resistance to Alternaria stem canker in tomato does not encode the enzyme aspartate carbamoyltransferase. Molec. Gen. Genet. 240, 43–48 (1993). https://doi.org/10.1007/BF00276882
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00276882